Page 945 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 945
Before we go into these reactions in detail, let us examine vinylcyclopropane itself, 929
which rearranges at high temperature to cyclopentene. 254
SECTION 10.6
Sigmatropic
Rearrangements
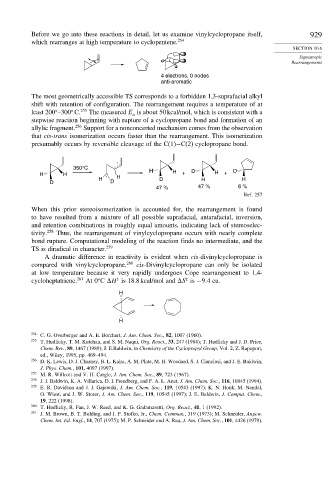
4 electrons, 0 nodes
anti-aromatic
The most geometrically accessible TS corresponds to a forbidden 1,3-suprafacial alkyl
shift with retention of configuration. The rearrangement requires a temperature of at
least 200 –300 C. 255 The measured E is about 50 kcal/mol, which is consistent with a
a
stepwise reaction beginning with rupture of a cyclopropane bond and formation of an
allylic fragment. 256 Support for a nonconcerted mechanism comes from the observation
that cis-trans isomerization occurs faster than the rearrangement. This isomerization
presumably occurs by reversible cleavage of the C(1)−C(2) cyclopropane bond.
.
350°C
H H . H H + D H + D
H H D H H
D D
47 % 47 % 6 %
Ref. 257
When this prior stereoisomerization is accounted for, the rearrangement is found
to have resulted from a mixture of all possible suprafacial, antarafacial, inversion,
and retention combinations in roughly equal amounts, indicating lack of stereoselec-
tivity. 258 Thus, the rearrangement of vinylcyclopropane occurs with nearly complete
bond rupture. Computational modeling of the reaction finds no intermediate, and the
TS is diradical in character. 259
A dramatic difference in reactivity is evident when cis-divinylcyclopropane is
compared with vinylcyclopropane. 260 cis-Divinylcyclopropane can only be isolated
at low temperature because it very rapidly undergoes Cope rearrangement to 1,4-
‡
‡
cycloheptatriene. 261 At 0 C H is 18.8 kcal/mol and S is −9 4 eu.
H
H
254
C. G. Overberger and A. E. Borchert, J. Am. Chem. Soc., 82, 1007 (1960).
255
T. Hudlicky, T. M. Kutchan, and S. M. Naqui, Org. React., 33, 247 (1984); T. Hudlicky and J. D. Price,
Chem. Rev., 89, 1467 (1989); J. E.Baldwin, in Chemistry of the Cyclopropyl Group, Vol. 2, Z. Rapoport,
ed., Wiley, 1995, pp. 469–494.
256 D. K. Lewis, D. J. Charney, B. L. Kalra, A. M. Plate, M. H. Woodard, S. J. Cianciosi, and J. E. Baldwin,
J. Phys. Chem., 101, 4097 (1997).
257 M. R. Willcott and V. H. Cargle, J. Am. Chem. Soc., 89, 723 (1967).
258
J. J. Baldwin, K. A. Villarica, D. I. Freedberg, and F. A. L. Anet, J. Am. Chem. Soc., 116, 10845 (1994).
259 E. R. Davidson and J. J. Gajewski, J. Am. Chem. Soc., 119, 10543 (1997); K. N. Houk, M. Nendal,
O. Wiest, and J. W. Storer, J. Am. Chem. Soc., 119, 10545 (1997); J. E. Baldwin, J. Comput. Chem.,
19, 222 (1998).
260 T. Hudlicky, R. Fan, J. W. Reed, and K. G. Gadamasetti, Org. React., 41, 1 (1992).
261
J. M. Brown, B. T. Bolding, and J. F. Stofko, Jr., Chem. Commun., 319 (1973); M. Schneider, Angew.
Chem. Int. Ed. Engl., 14, 707 (1975); M. P. Schneider and A. Rau, J. Am. Chem. Soc., 101, 4426 (1979).