Page 949 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 949
+0.06 +0.01 933
–0.17
–0.03 +0.26 –0.60
–0.03 6 SECTION 10.6
6 4 4
Sigmatropic
Rearrangements
1.967Å 1.967Å 3.219Å 2.310Å
–0.03
–0.26
1 3 3
–0.03 +0.06 1 +0.04
–0.29
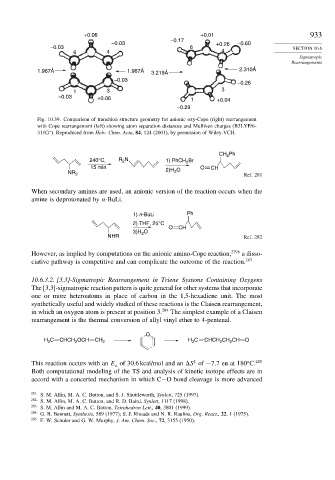
Fig. 10.39. Comparison of transition structure geometry for anionic oxy-Cope (right) rearrangement
with Cope rearrangement (left) showing atom separation distances and Mulliken charges (B3LYP/6-
311G ). Reproduced from Helv. Chim. Acta, 84, 124 (2001), by permission of Wiley-VCH.
∗
Ph
CH 2
240°C, R N 1) PhCH Br
2
2
15 min O CH
2
NR 2 2)H O Ref. 281
When secondary amines are used, an anionic version of the reaction occurs when the
amine is deprotonated by n-BuLi.
1) n-BuLi Ph
2) THF, 25°C
O CH
3)H O
2
NHR Ref. 282
However, as implied by computations on the anionic amino-Cope reaction, 279b a disso-
ciative pathway is competitive and can complicate the outcome of the reaction. 283
10.6.3.2. [3,3]-Sigmatropic Rearrangement in Triene Systems Containing Oxygens
The [3,3]-sigmatropic reaction pattern is quite general for other systems that incorporate
one or more heteroatoms in place of carbon in the 1,5-hexadiene unit. The most
synthetically useful and widely studied of these reactions is the Claisen rearrangement,
in which an oxygen atom is present at position 3. 284 The simplest example of a Claisen
rearrangement is the thermal conversion of allyl vinyl ether to 4-pentenal.
O
H C CHCH OCH CH 2 H C CHCH CH CH O
2
2
2
2
2
‡
This reaction occurs with an E of 30.6 kcal/mol and an S of −7 7euat180 C. 285
a
Both computational modeling of the TS and analysis of kinetic isotope effects are in
accord with a concerted mechanism in which C−O bond cleavage is more advanced
281 S. M. Allin, M. A. C. Button, and S. J. Shuttleworth, Synlett, 725 (1997).
282
S. M. Allin, M. A. C. Button, and R. D. Baird, Synlett, 1117 (1998).
283 S. M. Allin and M. A. C. Button, Tetrahedron Lett., 40, 3801 (1999).
284 G. B. Bennett, Synthesis, 589 (1977); S. J. Rhoads and N. R. Raulins, Org. React., 22, 1 (1975).
285
F. W. Schuler and G. W. Murphy, J. Am. Chem. Soc., 72, 3155 (1950).