Page 953 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 953
The decelerating effect of the 5-substituent is primarily on TS energy and is reflected 937
in the intrinsic barrier. Structurally, this may be due to a repulsive interaction between
the 5-oxy substituent and the ring oxygen. SECTION 10.6
Other donor substituents, e.g., trimethylsilyloxy, at C(2) are strongly accel- Sigmatropic
Rearrangements
erating. 301 This effect is the basis of the synthetic importance of ester enolate
Claisen rearrangements, in which enolates or silyl ketene acetals of allylic esters are
rearranged into 4-pentenoate esters. 302 This reaction is known as the Ireland-Claisen
rearrangement.
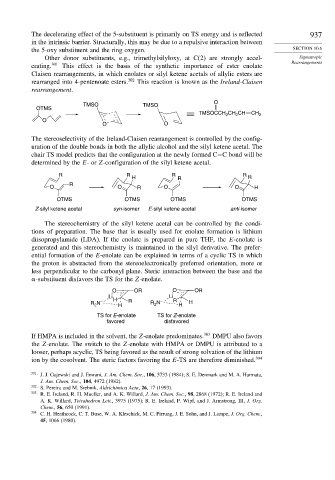
O
TMSO TMSO
OTMS
TMSOCCH CH CH CH 2
2
2
O
O O
The stereoselectivity of the Ireland-Claisen rearrangement is controlled by the config-
uration of the double bonds in both the allylic alcohol and the silyl ketene acetal. The
chair TS model predicts that the configuration at the newly formed C−C bond will be
determined by the E-or Z-configuration of the silyl ketene acetal.
R R R R
H R R
R
O O R O O H
OTMS OTMS OTMS OTMS
Z-silyl ketene acetal syn-isomer E-silyl ketene acetal anti-isomer
The stereochemistry of the silyl ketene acetal can be controlled by the condi-
tions of preparation. The base that is usually used for enolate formation is lithium
diisopropylamide (LDA). If the enolate is prepared in pure THF, the E-enolate is
generated and this stereochemistry is maintained in the silyl derivative. The prefer-
ential formation of the E-enolate can be explained in terms of a cyclic TS in which
the proton is abstracted from the stereoelectronically preferred orientation, more or
less perpendicular to the carbonyl plane. Steric interaction between the base and the
-substituent disfavors the TS for the Z-enolate.
O OR O OR
Li Li
R N – H H R R N – R H H
2
2
TS for E-enolate TS for Z-enolate
favored disfavored
If HMPA is included in the solvent, the Z-enolate predominates. 303 DMPU also favors
the Z-enolate. The switch to the Z-enolate with HMPA or DMPU is attributed to a
looser, perhaps acyclic, TS being favored as the result of strong solvation of the lithium
ion by the cosolvent. The steric factors favoring the E-TS are therefore diminished. 304
301 J. J. Gajewski and J. Emrani, J. Am. Chem. Soc., 106, 5733 (1984); S. E. Denmark and M. A. Harmata,
J. Am. Chem. Soc., 104, 4972 (1982).
302
S. Pereira and M. Srebnik, Aldrichimica Acta, 26, 17 (1993).
303 R. E. Ireland, R. H. Mueller, and A. K. Willard, J. Am. Chem. Soc., 98, 2868 (1972); R. E. Ireland and
A. K. Willard, Tetrahedron Lett., 3975 (1975); R. E. Ireland, P. Wipf, and J. Armstrong, III, J. Org.
Chem., 56, 650 (1991).
304
C. H. Heathcock, C. T. Buse, W. A. Kleschick, M. C. Pirrung, J. E. Sohn, and J. Lampe, J. Org. Chem.,
45, 1066 (1980).