Page 951 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 951
OH Ph CH 2 CH = CH 2 935
1
2
Ar OCH 2 CH = CHPh + Ar OCH 2 CH = CH 2 CHCH = CH 2 + OH
SECTION 10.6
1
2
Ar = phenyl Ar = 2 – naphthyl
Sigmatropic
31 32 33 34 Rearrangements
Ph
OH CHCH = CH 2
OH
CH 2 CH = CH 2
35 36
not formed not formed
The stereochemical features of the Claisen rearrangement are very similar to
those described for the Cope rearrangement, and stereochemical predictions can be
made on the basis of the preference for a chairlike TS. The major product has the
E-configuration at the newly formed double bond because of the preference for placing
the larger substituent in the pseudoequatorial position in the TS. 294
CH 3
H
CH 3 CH
CH 3
(CH 3 ) 2 CHCH 2
O O CH 3
CH 3 (CH 3 ) 2 CHCH 2
O CH 3 (CH 3 ) 2 CHCH 2
H H
Studies of chiral substrates have also demonstrated that chirality is maintained in the
reaction. 295 Examples of the synthetic application of the Claisen rearrangement are
discussed in Section 6.4.2.1 of Part B.
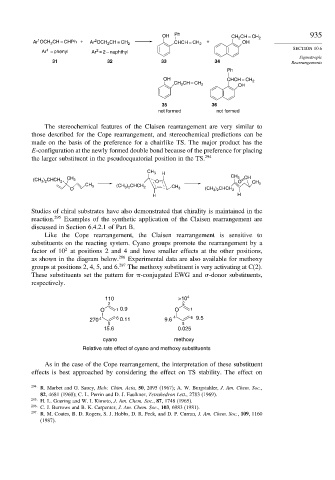
Like the Cope rearrangement, the Claisen rearrangement is sensitive to
substituents on the reacting system. Cyano groups promote the rearrangement by a
2
factor of 10 at positions 2 and 4 and have smaller effects at the other positions,
as shown in the diagram below. 296 Experimental data are also available for methoxy
groups at positions 2, 4, 5, and 6. 297 The methoxy substituent is very activating at C(2).
These substituents set the pattern for -conjugated EWG and -donor substituents,
respectively.
110 >10 4
2 2
O 1 0.9 O 1
270 4 6 0.11 9.6 4 6 9.5
5 5
15.6 0.025
cyano methoxy
Relative rate effect of cyano and methoxy substituents
As in the case of the Cope rearrangement, the interpretation of these substituent
effects is best approached by considering the effect on TS stability. The effect on
294
R. Marbet and G. Saucy, Helv. Chim. Acta, 50, 2095 (1967); A. W. Burgstahler, J. Am. Chem. Soc.,
82, 4681 (1960); C. L. Perrin and D. J. Faulkner, Tetrahedron Lett., 2783 (1969).
295 H. L. Goering and W. I. Kimoto, J. Am. Chem. Soc., 87, 1748 (1965).
296 C. J. Burrows and B. K. Carpenter, J. Am. Chem. Soc., 103, 6983 (1981).
297
R. M. Coates, B. D. Rogers, S. J. Hobbs, D. R. Peck, and D. P. Curran, J. Am. Chem. Soc., 109, 1160
(1987).