Page 946 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 946
930 Owing to unfavorable molecular geometry, the corresponding rearrangement
of trans-divinylcyclopropane to cycloheptatriene cannot be concerted and requires
CHAPTER 10 temperatures on the order of 190 C. The very low energy requirement for the Cope
Concerted Pericyclic rearrangement of cis-divinylcyclopropane reflects several favorable circumstances.
Reactions
The cis-orientation facilitates interaction of the diene termini, so the loss in entropy
in going to the TS is smaller than for an acyclic diene. The breaking bond is
strained and this reduces the E . The importance of the latter factor can be appre-
a
ciated by comparison with cis-divinylcyclobutane and cis-divinylcyclopentane. The
‡
former compound has H = 23 kcal/mol for rearrangement to cyclooctadiene. 262 cis-
Divinylcyclopentane does not rearrange to cyclononadiene, even at 250 C. 263 In the
latter case, the rearrangement is presumably thermodynamically unfavorable, since
there is no strain release from ring opening.
Divinylcyclopropane rearrangements can proceed with even greater ease if the
‡
S is made less negative by incorporating both vinyl groups into a ring. An example
of this is found in the degenerate homotropilidene rearrangement. A degenerate
rearrangement is a reaction process in which no overall change in structure occurs,
and the product of rearrangement is structurally identical to the starting material.
Depending on the rate at which the reaction occurs, the existence of a degenerate
rearrangement can be detected by use of isotopic labels or by interpretation of the
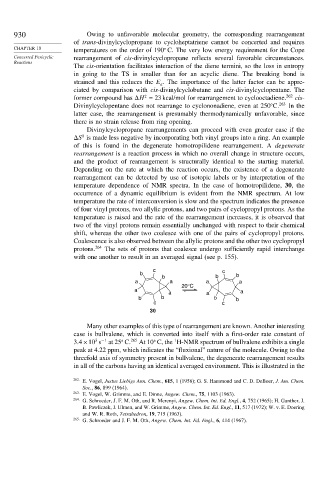
temperature dependence of NMR spectra. In the case of homotropilidene, 30, the
occurrence of a dynamic equilibrium is evident from the NMR spectrum. At low
temperature the rate of interconversion is slow and the spectrum indicates the presence
of four vinyl protons, two allylic protons, and two pairs of cyclopropyl protons. As the
temperature is raised and the rate of the rearrangement increases, it is observed that
two of the vinyl protons remain essentially unchanged with respect to their chemical
shift, whereas the other two coalesce with one of the pairs of cyclopropyl protons.
Coalescence is also observed between the allylic protons and the other two cyclopropyl
protons. 264 The sets of protons that coalesce undergo sufficiently rapid interchange
with one another to result in an averaged signal (see p. 155).
c c
b b
b b
a a a a
20°C
a a
a a
b b b b
c c
30
Many other examples of this type of rearrangement are known. Another interesting
case is bullvalene, which is converted into itself with a first-order rate constant of
1
3 −1
3 4×10 s at 25 C. 265 At 10 C, the H-NMR spectrum of bullvalene exhibits a single
peak at 4.22 ppm, which indicates the “fluxional” nature of the molecule. Owing to the
threefold axis of symmetry present in bullvalene, the degenerate rearrangement results
in all of the carbons having an identical averaged environment. This is illustrated in the
262
E. Vogel, Justus Liebigs Ann. Chem., 615, 1 (1958); G. S. Hammond and C. D. DeBoer, J. Am. Chem.
Soc., 86, 899 (1964).
263 E. Vogel, W. Grimme, and E. Dinne, Angew. Chem., 75, 1103 (1963).
264 G. Schroeder, J. F. M. Oth, and R. Merenyi, Angew. Chem. Int. Ed. Engl., 4, 752 (1965); H. Gunther, J.
B. Pawliczek, J. Ulmen, and W. Grimme, Angew. Chem. Int. Ed. Engl., 11, 517 (1972); W. v. E. Doering
and W. R. Roth, Tetrahedron, 19, 715 (1963).
265
G. Schroeder and J. F. M. Oth, Angew. Chem. Int. Ed. Engl., 6, 414 (1967).