Page 943 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 943
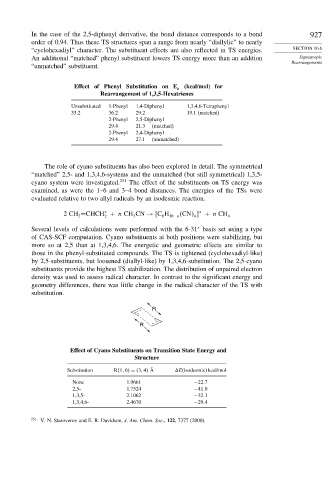
In the case of the 2,5-diphenyl derivative, the bond distance corresponds to a bond 927
order of 0.94. Thus these TS structures span a range from nearly “diallylic” to nearly
“cyclohexadiyl” character. The substituent effects are also reflected in TS energies. SECTION 10.6
An additional “matched” phenyl substituent lowers TS energy more than an addition Sigmatropic
Rearrangements
“unmatched” substituent.
Effect of Phenyl Substitution on E (kcal/mol) for
a
Rearrangement of 1,3,5-Hexatrienes
Unsubstituted 1-Phenyl 1,4-Diphenyl 1,3,4,6-Tetraphenyl
33.2 36.2 29.2 19.1 (matched)
2-Phenyl 2,5-Diphenyl
29.4 21.3 (matched)
2-Phenyl 2,4-Diphenyl
29.4 27.1 (unmatched)
The role of cyano substituents has also been explored in detail. The symmetrical
“matched” 2,5- and 1,3,4,6-systems and the unmatched (but still symmetrical) 1,3,5-
cyano system were investigated. 251 The effect of the substituents on TS energy was
examined, as were the 1–6 and 3–4 bond distances. The energies of the TSs were
evaluated relative to two allyl radicals by an isodesmic reaction.
∗
∗
2CH =CHCH + n CH CN → C H 10−n CN + n CH 4
3
n
2
2
6
∗
Several levels of calculations were performed with the 6-31 basis set using a type
of CAS-SCF computation. Cyano substituents at both positions were stabilizing, but
more so at 2,5 than at 1,3,4,6. The energetic and geometric effects are similar to
those in the phenyl-substituted compounds. The TS is tightened (cyclohexadiyl-like)
by 2,5-substituents, but loosened (diallyl-like) by 1,3,4,6-substitution. The 2,5-cyano
substituents provide the highest TS stabilization. The distribution of unpaired electron
density was used to assess radical character. In contrast to the significant energy and
geometry differences, there was little change in the radical character of the TS with
substitution.
R
R
Effect of Cyano Substituents on Transition State Energy and
Structure
Substitution R 1 6 = 3 4 Å E(isodesmic) kcal/mol
None 1.9661 −22 7
2,5- 1.7524 −41 0
1,3,5- 2.1062 −32 1
1,3,4,6- 2.4670 −29 4
251
V. N. Staroverov and E. R. Davidson, J. Am. Chem. Soc., 122, 7377 (2000).