Page 950 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 950
934 than C−C bond formation. 286 Claisen rearrangements show a considerable sensitivity
to solvent polarity, with reaction rates increasing with solvent polarity. 287 Water is an
CHAPTER 10 288
especially favorable solvent. The solvent effect is believed to be due to differential
Concerted Pericyclic solvation of the reactants and TS. Hydrogen bonding contributes to stabilization of the
Reactions
TS. 289
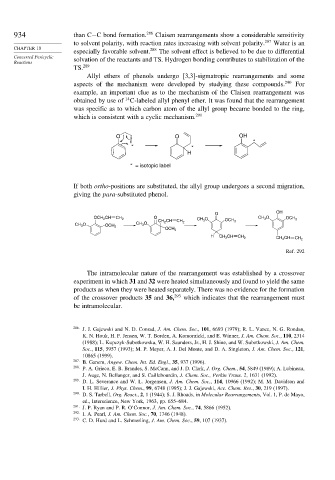
Allyl ethers of phenols undergo [3,3]-sigmatropic rearrangements and some
aspects of the mechanism were developed by studying these compounds. 290 For
example, an important clue as to the mechanism of the Claisen rearrangement was
14
obtained by use of C-labeled allyl phenyl ether. It was found that the rearrangement
was specific as to which carbon atom of the allyl group became bonded to the ring,
which is consistent with a cyclic mechanism. 291
O O OH
*
* *
H
* = isotopic label
If both ortho-positions are substituted, the allyl group undergoes a second migration,
giving the para-substituted phenol.
O OH
OCH 2 CH CH 2 O CH 3 O CH 3 O OCH 3
CH 2 CH CH 2 OCH 3
CH 3 O OCH 3 CH 3 O
OCH 3
H CH 2 CH CH 2 CH 2 CH CH 2
Ref. 292
The intramolecular nature of the rearrangement was established by a crossover
experiment in which 31 and 32 were heated simultaneously and found to yield the same
products as when they were heated separately. There was no evidence for the formation
of the crossover products 35 and 36, 293 which indicates that the rearrangement must
be intramolecular.
286
J. J. Gajewski and N. D. Conrad, J. Am. Chem. Soc., 101, 6693 (1979); R. L. Vance, N. G. Rondan,
K. N. Houk, H. F. Jensen, W. T. Borden, A. Komornicki, and E. Winner, J. Am. Chem. Soc., 110, 2314
(1988); L. Kupczyk-Subotkowska, W. H. Saunders, Jr., H. J. Shine, and W. Subotkowski, J. Am. Chem.
Soc., 115, 5957 (1993); M. P. Meyer, A. J. Del Monte, and D. A. Singleton, J. Am. Chem. Soc., 121,
10865 (1999).
287 B. Ganem, Angew. Chem. Int. Ed. Engl., 35, 937 (1996).
288
P. A. Grieco, E. B. Brandes, S. McCann, and J. D. Clark, J. Org. Chem., 54, 5849 (1989); A. Lubineau,
J. Auge, N. Bellanger, and S. Caillebourdin, J. Chem. Soc., Perkin Trans. 2, 1631 (1992).
289 D. L. Severance and W. L. Jorgensen, J. Am. Chem. Soc., 114, 10966 (1992); M. M. Davidson and
I. H. Hillier, J. Phys. Chem., 99, 6748 (1995); J. J. Gajewski, Acc. Chem. Res., 30, 219 (1997).
290 D. S. Tarbell, Org. React., 2, 1 (1944); S. J. Rhoads, in Molecular Rearrangements, Vol. 1, P. de Mayo,
ed., Interscience, New York, 1963, pp. 655–684.
291
J. P. Ryan and P. R. O’Connor, J. Am. Chem. Soc., 74, 5866 (1952).
292 I. A. Pearl, J. Am. Chem. Soc., 70, 1746 (1948).
293
C. D. Hurd and L. Schmerling, J. Am. Chem. Soc., 59, 107 (1937).