Page 954 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 954
938 These general principles of solvent control of enolate stereochemistry are applicable
to other systems. 305
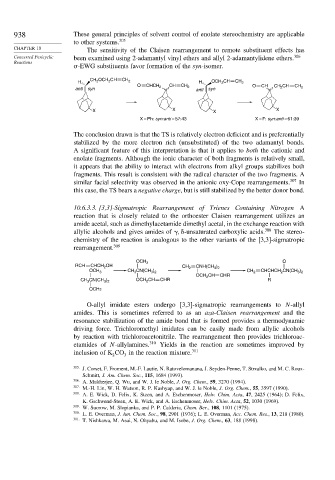
CHAPTER 10 The sensitivity of the Claisen rearrangement to remote substituent effects has
Concerted Pericyclic been examined using 2-adamantyl vinyl ethers and allyl 2-adamantylidene ethers. 306
Reactions
-EWG substituents favor formation of the syn-isomer.
CH 2 OCH 2 CH
H CH 2 H OCH 2 CH CH 2
O CHCH 2 CH CH 2 O CH CH 2 CH
anti syn anti syn CH 2
X X X X
X = Ph: syn:anti = 57:43 X = F: syn:anti = 61:39
The conclusion drawn is that the TS is relatively electron deficient and is preferentially
stabilized by the more electron rich (unsubstituted) of the two adamantyl bonds.
A significant feature of this interpretation is that it applies to both the cationic and
enolate fragments. Although the ionic character of both fragments is relatively small,
it appears that the ability to interact with electrons from alkyl groups stabilizes both
fragments. This result is consistent with the radical character of the two fragments. A
similar facial selectivity was observed in the anionic oxy-Cope rearrangements. 307 In
this case, the TS bears a negative charge, but is still stabilized by the better donor bond.
10.6.3.3. [3,3]-Sigmatropic Rearrangement of Trienes Containing Nitrogen A
reaction that is closely related to the orthoester Claisen rearrangement utilizes an
amide acetal, such as dimethylacetamide dimethyl acetal, in the exchange reaction with
allylic alcohols and gives amides of -unsaturated carboxylic acids. 308 The stereo-
chemistry of the reaction is analogous to the other variants of the [3,3]-sigmatropic
rearrangement. 309
O
OCH 3
RCH CHCH 2 OH CH 2 CNH(CH 3 ) 2
OCH 3 CH 3 CN(CH 3 ) 2 CH 2 CHCHCH 2 CN(CH 3 ) 2
OCH 2 CH CHR
OCH 2 CH CHR R
CH 3 CN(CH 3 ) 2
OCH3
O-allyl imidate esters undergo [3,3]-sigmatropic rearrangements to N-allyl
amides. This is sometimes referred to as an aza-Claisen rearrangement and the
resonance stabilization of the amide bond that is formed provides a thermodynamic
driving force. Trichloromethyl imidates can be easily made from allylic alcohols
by reaction with trichloroacetonitrile. The rearrangement then provides trichloroac-
etamides of N-allylamines. 310 Yields in the reaction are sometimes improved by
inclusion of K CO in the reaction mixture. 311
2
3
305 J. Corset, F. Froment, M.-F. Lautie, N. Ratovelomanana, J. Seyden-Penne, T. Strzalko, and M. C. Roux-
Schmitt, J. Am. Chem. Soc., 115, 1684 (1993).
306
A. Mukherjee, Q. Wu, and W. J. le Noble, J. Org. Chem., 59, 3270 (1994).
307 M.-H. Lin, W. H. Watson, R. P. Kashyap, and W. J. le Noble, J. Org. Chem., 55, 3597 (1990).
308 A. E. Wick, D. Felix, K. Steen, and A. Eschenmoser, Helv. Chim. Acta, 47, 2425 (1964); D. Felix,
K. Gschwend-Steen, A. E. Wick, and A. Eschenmoser, Helv. Chim. Acta, 52, 1030 (1969).
309
W. Sucrow, M. Slopianka, and P. P. Calderia, Chem. Ber., 108, 1101 (1975).
310 L. E. Overman, J. Am. Chem. Soc., 98, 2901 (1976); L. E. Overman, Acc. Chem. Res., 13, 218 (1980).
311
T. Nishkawa, M. Asai, N. Ohyabu, and M. Isobe, J. Org. Chem., 63, 188 (1998).