Page 936 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 936
920 The TS for [3,3]-sigmatropic rearrangements can be considered to be two interacting
allyl fragments. When the process is suprafacial in both groups, an aromatic orbital
CHAPTER 10
array results and the process is thermally allowed. Usually a chairlike TS is involved
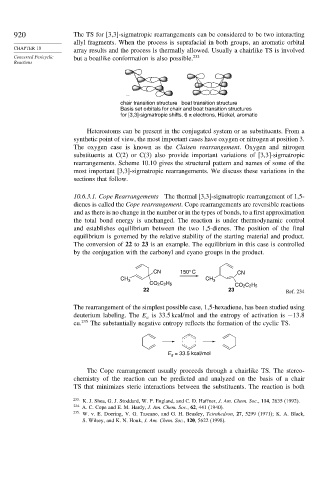
Concerted Pericyclic but a boatlike conformation is also possible. 233
Reactions
chair transition structure boat transition structure
Basis set orbitals for chair and boat transition structures
for [3,3]-sigmatropic shifts. 6 π electrons, Hückel, aromatic
Heteroatoms can be present in the conjugated system or as substituents. From a
synthetic point of view, the most important cases have oxygen or nitrogen at position 3.
The oxygen case is known as the Claisen rearrangement. Oxygen and nitrogen
substituents at C(2) or C(3) also provide important variations of [3,3]-sigmatropic
rearrangements. Scheme 10.10 gives the structural pattern and names of some of the
most important [3,3]-sigmatropic rearrangements. We discuss these variations in the
sections that follow.
10.6.3.1. Cope Rearrangements The thermal [3,3]-sigmatropic rearrangement of 1,5-
dienes is called the Cope rearrangement. Cope rearrangements are reversible reactions
and as there is no change in the number or in the types of bonds, to a first approximation
the total bond energy is unchanged. The reaction is under thermodynamic control
and establishes equilibrium between the two 1,5-dienes. The position of the final
equilibrium is governed by the relative stability of the starting material and product.
The conversion of 22 to 23 is an example. The equilibrium in this case is controlled
by the conjugation with the carbonyl and cyano groups in the product.
CN 150° C CN
CH 3 CH 3
CO 2C 2H 5
CO 2C 2H 5
22 23 Ref. 234
The rearrangement of the simplest possible case, 1,5-hexadiene, has been studied using
deuterium labeling. The E is 33.5 kcal/mol and the entropy of activation is −13 8
a
eu. 235 The substantially negative entropy reflects the formation of the cyclic TS.
E = 33.5 kcal/mol
a
The Cope rearrangement usually proceeds through a chairlike TS. The stereo-
chemistry of the reaction can be predicted and analyzed on the basis of a chair
TS that minimizes steric interactions between the substituents. The reaction is both
233 K. J. Shea, G. J. Stoddard, W. P. England, and C. D. Haffner, J. Am. Chem. Soc., 114, 2635 (1992).
234 A. C. Cope and E. M. Hardy, J. Am. Chem. Soc., 62, 441 (1940).
235
W. v. E. Doering, V. G. Tascano, and G. H. Beasley, Tetrahedron, 27, 5299 (1971); K. A. Black,
S. Wilsey, and K. N. Houk, J. Am. Chem. Soc., 120, 5622 (1998).