Page 933 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 933
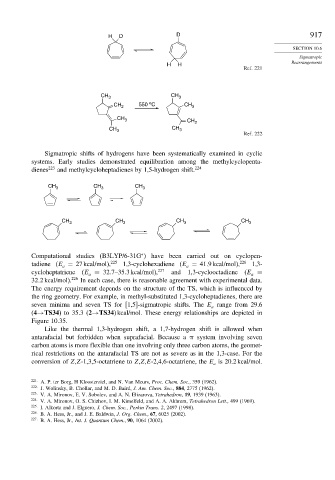
H D D 917
SECTION 10.6
Sigmatropic
Rearrangements
H H
Ref. 221
CH 3 CH 3
o
CH 2 550 C CH 3
CH 3 CH 2
CH 3 CH 3
Ref. 222
Sigmatropic shifts of hydrogens have been systematically examined in cyclic
systems. Early studies demonstrated equilibration among the methylcyclopenta-
dienes 223 and methylcycloheptadienes by 1,5-hydrogen shift. 224
CH 3 CH 3 CH 3
CH 3 CH 3 CH 3 CH 3
∗
Computational studies (B3LYP/6-31G ) have been carried out on cyclopen-
tadiene (E = 27 kcal/mol), 225 1,3-cyclohexadiene (E = 41 9 kcal/mol), 226 1,3-
a
a
cycloheptatriene (E = 32.7–35.3 kcal/mol), 227 and 1,3-cyclooctadiene (E =
a a
32 2 kcal/mol). 226 In each case, there is reasonable agreement with experimental data.
The energy requirement depends on the structure of the TS, which is influenced by
the ring geometry. For example, in methyl-substituted 1,3-cycloheptadienes, there are
seven minima and seven TS for [1,5]-sigmatropic shifts. The E range from 29.6
a
(4→TS34) to 35.3 (2→TS34) kcal/mol. These energy relationships are depicted in
Figure 10.35.
Like the thermal 1,3-hydrogen shift, a 1,7-hydrogen shift is allowed when
antarafacial but forbidden when suprafacial. Because a system involving seven
carbon atoms is more flexible than one involving only three carbon atoms, the geomet-
rical restrictions on the antarafacial TS are not as severe as in the 1,3-case. For the
conversion of Z,Z-1,3,5-octatriene to Z,Z,E-2,4,6-octatriene, the E is 20.2 kcal/mol.
a
221
A. P. ter Borg, H Kloosterziel, and N. Van Meurs, Proc. Chem. Soc., 359 (1962).
222
J. Wolinsky, B. Chollar, and M. D. Baird, J. Am. Chem. Soc., 884, 2775 (1962).
223 V. A. Mironov, E. V. Sobolev, and A. N. Elizarova, Tetrahedron, 19, 1939 (1963).
224
V. A. Mironov, O. S. Chizhov, I. M. Kimelfeld, and A. A. Akhrem, Tetrahedron Lett., 499 (1969).
225 I. Alkorta and J. Elguero, J. Chem. Soc., Perkin Trans. 2, 2497 (1998).
226 B. A. Hess, Jr., and J. E. Baldwin, J. Org. Chem., 67, 6025 (2002).
227
B. A. Hess, Jr., Int. J. Quantum Chem., 90, 1064 (2002).