Page 928 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 928
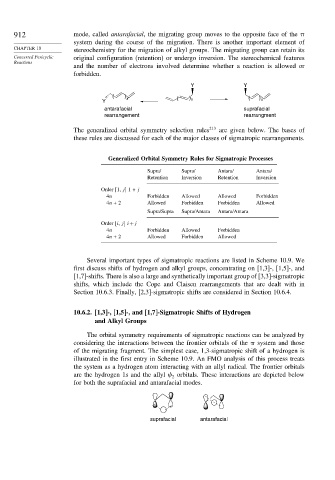
912 mode, called antarafacial, the migrating group moves to the opposite face of the
system during the course of the migration. There is another important element of
CHAPTER 10 stereochemistry for the migration of alkyl groups. The migrating group can retain its
Concerted Pericyclic original configuration (retention) or undergo inversion. The stereochemical features
Reactions
and the number of electrons involved determine whether a reaction is allowed or
forbidden.
Y Y
( ) x ( ( )
Y ) x x
antarafacial suprafacial
rearrangement rearrangment
The generalized orbital symmetry selection rules 213 are given below. The bases of
these rules are discussed for each of the major classes of sigmatropic rearrangements.
Generalized Orbital Symmetry Rules for Sigmatropic Processes
Supra/ Supra/ Antara/ Antara/
Retention Inversion Retention Inversion
Order [1, j]1+ j
4n Forbidden Allowed Allowed Forbidden
4n + 2 Allowed Forbidden Forbidden Allowed
Supra/Supra Supra/Antara Antara/Antara
Order [i, j] i+j
4n Forbidden Allowed Forbidden
4n + 2 Allowed Forbidden Allowed
Several important types of sigmatropic reactions are listed in Scheme 10.9. We
first discuss shifts of hydrogen and alkyl groups, concentrating on [1,3]-, [1,5]-, and
[1,7]-shifts. There is also a large and synthetically important group of [3,3]-sigmatropic
shifts, which include the Cope and Claisen rearrangements that are dealt with in
Section 10.6.3. Finally, [2,3]-sigmatropic shifts are considered in Section 10.6.4.
10.6.2. [1,3]-, [1,5]-, and [1,7]-Sigmatropic Shifts of Hydrogen
and Alkyl Groups
The orbital symmetry requirements of sigmatropic reactions can be analyzed by
considering the interactions between the frontier orbitals of the system and those
of the migrating fragment. The simplest case, 1,3-sigmatropic shift of a hydrogen is
illustrated in the first entry in Scheme 10.9. An FMO analysis of this process treats
the system as a hydrogen atom interacting with an allyl radical. The frontier orbitals
are the hydrogen 1s and the allyl
orbitals. These interactions are depicted below
2
for both the suprafacial and antarafacial modes.
suprafacial antarafacial