Page 927 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 927
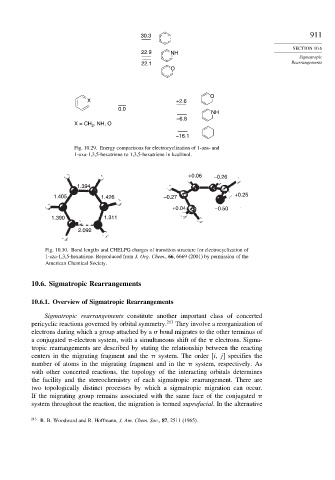
30.3 911
SECTION 10.6
22.9 NH
Sigmatropic
22.1 Rearrangements
O
O
X +2.6
0.0
NH
–6.8
, NH, O
X = CH 2
–16.1
Fig. 10.29. Energy comparisons for electrocyclization of 1-aza- and
1-oxa-1,3,5-hexatriene to 1,3,5-hexatriene in kcal/mol.
+0.06 –0.26
1.394
1.405 1.426 –0.27 +0.25
+0.04 –0.50
1.390 1.311
2.092
Fig. 10.30. Bond lengths and CHELPG charges of transition structure for electrocyclization of
1-aza-1,3,5-hexatriene. Reproduced from J. Org. Chem., 66, 6669 (2001) by permission of the
American Chemical Society.
10.6. Sigmatropic Rearrangements
10.6.1. Overview of Sigmatropic Rearrangements
Sigmatropic rearrangements constitute another important class of concerted
pericyclic reactions governed by orbital symmetry. 213 They involve a reorganization of
electrons during which a group attached by a bond migrates to the other terminus of
a conjugated -electron system, with a simultaneous shift of the electrons. Sigma-
tropic rearrangements are described by stating the relationship between the reacting
centers in the migrating fragment and the system. The order [i, j] specifies the
number of atoms in the migrating fragment and in the system, respectively. As
with other concerted reactions, the topology of the interacting orbitals determines
the facility and the stereochemistry of each sigmatropic rearrangement. There are
two topologically distinct processes by which a sigmatropic migration can occur.
If the migrating group remains associated with the same face of the conjugated
system throughout the reaction, the migration is termed suprafacial. In the alternative
213
R. B. Woodward and R. Hoffmann, J. Am. Chem. Soc., 87, 2511 (1965).