Page 922 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 922
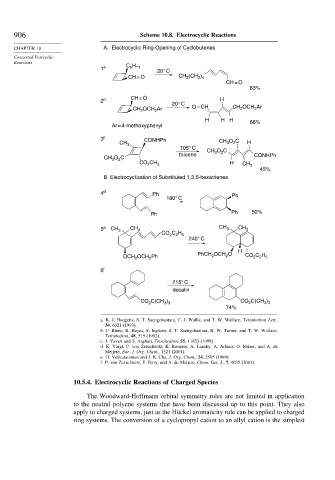
906 Scheme 10.8. Electrocyclic Reactions
CHAPTER 10 A. Electrocyclic Ring-Opening of Cyclobutenes
Concerted Pericyclic
Reactions
5 11
1 a C H
20° C
CH = O CH 3 (CH )
2 4
CH = O
83%
2 b CH = O H
20° C
CH 2 OCH 2 Ar O = CH CH OCH Ar
2
2
H H H 86%
Ar = 4-methoxyphenyl
3 c CONHPh CH O C
CH 3 3 2 H
105° C
CH 3 O C
2
toluene CONHPh
CH O C
3
2
CO 2 CH 3 H CH 3
45%
B. Electrocyclization of Substituted 1,3,5-hexatrienes.
4 d Ph Ph
180° C
Ph Ph 50%
5 e CH 3 CH 3 CH 3 CH 3
CO C H
2 2 5
240° C
H
OCH O CO C H
OCH OCH Ph PhCH 2 2 2 2 5
2
2
6 f
215° C
decalin
C(CH ) CO C(CH )
CO 2 3 3 2 3 3
74%
a. K. J. Hodgetts, S. T. Saengchantara, C. J. Wallis, and T. W. Wallace, Tetrahedron Lett.,
34, 6321 (1993).
b. F. Binns, R. Hayes, S. Ingham, S. T. Saengchantara, R. W. Turner, and T. W. Wallace,
Tetrahedron, 48, 515 (1992).
c. I. Yavari and S. Asghari, Tetrahedron, 55, 11853 (1999).
d. K. Voigt, P. von Zezschwitz, K. Rosauer, A. Lansky, A. Adams, O. Reiser, and A. de
Meijere, Eur. J. Org. Chem., 1521 (2001).
e. H. Venkataraman and J. K. Cha, J. Org. Chem., 54, 2505 (1989).
f. P. von Zezschwitz, F. Petry, and A. de Meijere, Chem. Eur. J., 7, 4035 (2001).
10.5.4. Electrocyclic Reactions of Charged Species
The Woodward-Hoffmann orbital symmetry rules are not limited in application
to the neutral polyene systems that have been discussed up to this point. They also
apply to charged systems, just as the Hückel aromaticity rule can be applied to charged
ring systems. The conversion of a cyclopropyl cation to an allyl cation is the simplest