Page 920 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 920
904 The unsubstituted Dewar benzene 10 was successfully prepared in 1963.
CHAPTER 10 O
H H
Concerted Pericyclic Pb(OAc) 4
Reactions O hv O
H O
H O 10
O
This compound is less stable than 9 and reverts to benzene with a half-life of about
‡
2 days at 25 C, with H = 23 kcal/mol. 189 Nevertheless, the relative kinetic stability
of Dewar benzene is surprisingly high when one considers that its conversion to
benzene is exothermic by 71 kcal/mol. Furthermore, the central bond is not only
strained but also bis-allylic. The kinetic stability of Dewar benzene is related to
the orbital symmetry requirements for concerted electrocyclic transformations. The
concerted thermal pathway would be conrotatory, since the reaction is the ring opening
of a cyclobutene and therefore leads not to benzene, but to a highly strained Z,Z,E-
cyclohexatriene. A disrotatory process, which would lead directly to benzene, is
forbidden.
H
H H
H
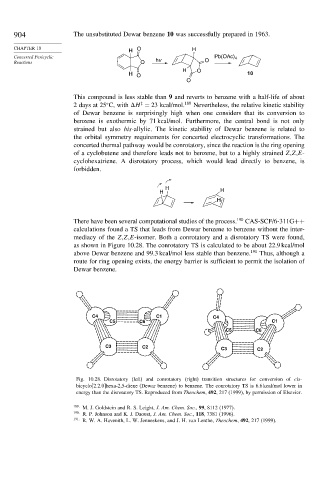
There have been several computational studies of the process. 190 CAS-SCF/6-311G++
calculations found a TS that leads from Dewar benzene to benzene without the inter-
mediacy of the Z,Z,E-isomer. Both a conrotatory and a disrotatory TS were found,
as shown in Figure 10.28. The conrotatory TS is calculated to be about 22.9 kcal/mol
above Dewar benzene and 99.3 kcal/mol less stable than benzene. 191 Thus, although a
route for ring opening exists, the energy barrier is sufficient to permit the isolation of
Dewar benzene.
C4 C1 C4
C5 C6 C1
C5 C6
C3 C2 C3 C2
Fig. 10.28. Disrotatory (left) and conrotatory (right) transition structures for conversion of cis-
bicyclo[2.2.0]hexa-2,5-diene (Dewar benzene) to benzene. The conrotatory TS is 6.6 kcal/mol lower in
energy than the disrotatory TS. Reproduced from Theochem, 492, 217 (1999), by permission of Elsevier.
189
M. J. Goldstein and R. S. Leight, J. Am. Chem. Soc., 99, 8112 (1977).
190 R. P. Johnson and K. J. Daoust, J. Am. Chem. Soc., 118, 7381 (1996).
191
R. W. A. Havenith, L. W. Jenneskens, and J. H. van Lenthe, Theochem, 492, 217 (1999).