Page 918 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 918
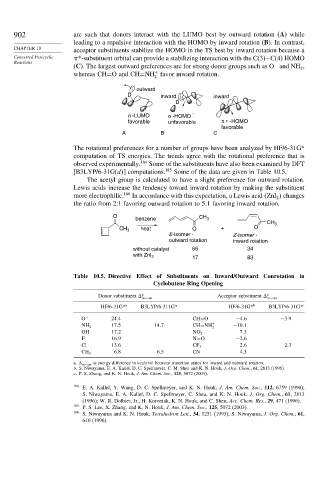
902 are such that donors interact with the LUMO best by outward rotation (A) while
leading to a repulsive interaction with the HOMO by inward rotation (B). In contrast,
CHAPTER 10
acceptor substituents stabilize the HOMO in the TS best by inward rotation because a
Concerted Pericyclic *-substituent orbital can provide a stabilizing interaction with the C(3)−C(4) HOMO
Reactions
−
(C). The largest outward preferences are for strong donor groups such as O and NH ,
2
whereas CH=O and CH=NH favor inward rotation.
+
2
: outward
D inward : inward
D
σ -LUMO σ -HOMO
favorable unfavorable π ∗ -HOMO
favorable
A B C
The rotational preferences for a number of groups have been analyzed by HF/6-31G*
computation of TS energies. The trends agree with the rotational preference that is
observed experimentally. 184 Some of the substituents have also been examined by DFT
[B3LYP/6-31G(d ] computations. 185 Some of the data are given in Table 10.5.
The acetyl group is calculated to have a slight preference for outward rotation.
Lewis acids increase the tendency toward inward rotation by making the substituent
more electrophilic. 186 In accordance with this expectation, a Lewis acid ZnI changes
2
the ratio from 2:1 favoring outward rotation to 5:1 favoring inward rotation.
O
benzene CH 3
CH 3
CH 3 heat O + O
E-isomer - Z-isomer -
outward rotation inward rotation
without catalyst 66 34
with ZnI 2 17 83
Table 10.5. Directive Effect of Substituents on Inward/Outward Conrotation in
Cyclobutene Ring Opening
Donor substituent a Acceptor substituent a
in−out in−out
HF/6-31G* a B3LYP/6-311G* HF/6-31G* b B3LYP/6-31G* c
O − 24 4 CH=O −4 6 −3 9
17 5 14 7 CH=NH + −10 1
NH 2
2
OH 17 2 NO 2 7 3
F 16 9 N=O −2 6
Cl 13 6 CF 3 2 6 2 3
6 8 6 5 CN 4 3
CH 3
a. in−out is energy difference in kcal/mol between transition states for inward and outward rotation.
b. S. Niwayama, E. A. Kallel, D. C. Spellmeyer, C. M. Sheu and K. N. Houk, J. Org. Chem., 61, 2813 (1996).
c. P. S. Zhang, and K. N. Houk, J. Am. Chem. Soc., 125, 5072 (2003).
184 E. A. Kallel, Y. Wang, D. C. Spellmeyer, and K. N. Houk, J. Am. Chem. Soc., 112, 6759 (1990);
S. Niwayama, E. A. Kallel, D. C. Spellmeyer, C. Sheu, and K. N. Houk, J. Org. Chem., 61, 2813
(1996); W. R. Dolbier, Jr., H. Koroniak, K. N. Houk, and C. Sheu, Acc. Chem. Res., 29, 471 (1996).
185 P. S. Lee, X. Zhang, and K. N. Houk, J. Am. Chem. Soc., 125, 5072 (2003).
186
S. Niwayama and K. N. Houk, Tetrahedron Lett., 34, 1251 (1993); S. Niwayama, J. Org. Chem., 61,
640 (1996).