Page 917 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 917
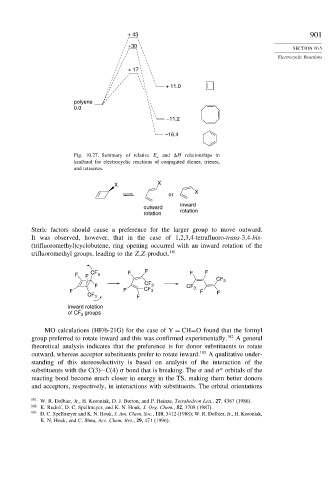
+ 43 901
+30
SECTION 10.5
Electrocyclic Reactions
+ 17
+ 11.0
polyene
0.0
–11.2
–16.4
Fig. 10.27. Summary of relative E a and H relationships in
kcal/mol for electrocyclic reactions of conjugated dienes, trienes,
and tetraenes.
X X
X
or
inward
outward
rotation rotation
Steric factors should cause a preference for the larger group to move outward.
It was observed, however, that in the case of 1,2,3,4-tetrafluoro-trans-3,4-bis-
(trifluoromethyl)cyclobutene, ring opening occurred with an inward rotation of the
trifluoromethyl groups, leading to the Z,Z-product. 181
F F CF 3 F F F F
CF 3
CF
F 3 CF
F F CF 3 3 F F
CF 3 F
inward rotation
of CF 3 groups
MO calculations (HF/6-21G) for the case of Y = CH=O found that the formyl
group preferred to rotate inward and this was confirmed experimentally. 182 A general
theoretical analysis indicates that the preference is for donor substituents to rotate
outward, whereas acceptor substituents prefer to rotate inward. 183 A qualitative under-
standing of this stereoselectivity is based on analysis of the interaction of the
substituents with the C(3)−C(4) bond that is breaking. The and * orbitals of the
reacting bond become much closer in energy in the TS, making them better donors
and acceptors, respectively, in interactions with substituents. The orbital orientations
181
W. R. Dolbier, Jr., H. Koroniak, D. J. Burton, and P. Heinze, Tetrahedron Lett., 27, 4387 (1986).
182 K. Rudolf, D. C. Spellmeyer, and K. N. Houk, J. Org. Chem., 52, 3708 (1987).
183
D. C. Spellmeyer and K. N. Houk, J. Am. Chem. Soc., 110, 3412 (1988); W. R. Dolbier, Jr., H. Koroniak,
K. N. Houk, and C. Sheu, Acc. Chem. Res., 29, 471 (1996).