Page 925 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 925
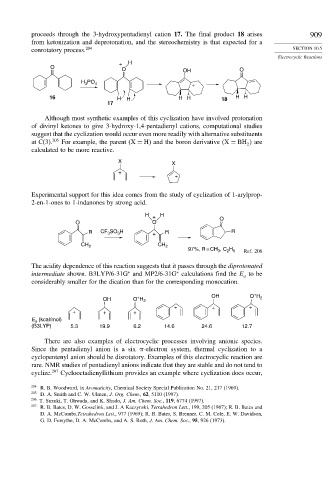
proceeds through the 3-hydroxypentadienyl cation 17. The final product 18 arises 909
from ketonization and deprotonation, and the stereochemistry is that expected for a
conrotatory process. 204 SECTION 10.5
Electrocyclic Reactions
+ H
O
O OH O
H PO
3
4
+
16 H H HH 18 HH
17
Although most synthetic examples of this cyclization have involved protonation
of divinyl ketones to give 3-hydroxy-1,4-pentadienyl cations, computational studies
suggest that the cyclization would occur even more readily with alternative substituents
at C(3). 205 For example, the parent (X = H) and the boron derivative (X = BH are
2
calculated to be more reactive.
X X
+
+
Experimental support for this idea comes from the study of cyclization of 1-arylprop-
2-en-1-ones to 1-indanones by strong acid.
H + H
O O O
R CF SO H + R R
3
3
CH 2 CH 2
97%, R = CH , C H Ref. 206
3
2 5
The acidity dependence of this reaction suggests that it passes through the diprotonated
∗
∗
intermediate shown. B3LYP/6-31G and MP2/6-31G calculations find the E to be
a
considerably smaller for the dication than for the corresponding monocation.
+
+
OH O H 2 OH O H 2
+ + +
+ + +
E (kcal/mol)
a
(B3LYP) 5.3 19.9 6.2 14.6 24.6 12.7
There are also examples of electrocyclic processes involving anionic species.
Since the pentadienyl anion is a six -electron system, thermal cyclization to a
cyclopentenyl anion should be disrotatory. Examples of this electrocyclic reaction are
rare. NMR studies of pentadienyl anions indicate that they are stable and do not tend to
cyclize. 207 Cyclooctadienyllithium provides an example where cyclization does occur,
204
R. B. Woodward, in Aromaticity, Chemical Society Special Publication No. 21, 217 (1969).
205
D. A. Smith and C. W. Ulmen, J. Org. Chem., 62, 5110 (1997).
206 T. Suzuki, T. Ohwada, and K. Shudo, J. Am. Chem. Soc., 119, 6774 (1997).
207
R. B. Bates, D. W. Gosselink, and J. A Kaczynski, Tetrahedron Lett., 199, 205 (1967); R. B. Bates and
D. A. McCombs,Tetrahedron Lett., 977 (1969); R. B. Bates, S. Brenner, C. M. Cole, E. W. Davidson,
G. D. Forsythe, D. A. McCombs, and A. S. Roth, J. Am. Chem. Soc., 95, 926 (1973).