Page 930 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 930
914 Orbital symmetry analysis of the 1,5-sigmatropic shift of hydrogen leads to the
opposite conclusion. The relevant frontier orbitals in this case are the hydrogen 1s
CHAPTER 10 and
of the pentadienyl radical. The suprafacial mode is allowed, whereas the
3
Concerted Pericyclic antarafacial mode is forbidden. The suprafacial shift corresponds to a geometrically
Reactions
favorable six-membered ring.
allowed [1,5]-suprafacial
hydrogen shift in 1,3-pentadiene
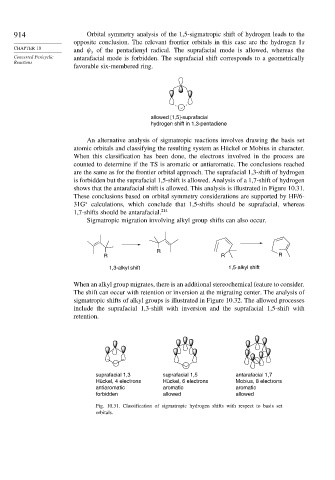
An alternative analysis of sigmatropic reactions involves drawing the basis set
atomic orbitals and classifying the resulting system as Hückel or Mobius in character.
When this classification has been done, the electrons involved in the process are
counted to determine if the TS is aromatic or antiaromatic. The conclusions reached
are the same as for the frontier orbital approach. The suprafacial 1,3-shift of hydrogen
is forbidden but the suprafacial 1,5-shift is allowed. Analysis of a 1,7-shift of hydrogen
shows that the antarafacial shift is allowed. This analysis is illustrated in Figure 10.31.
These conclusions based on orbital symmetry considerations are supported by HF/6-
∗
31G calculations, which conclude that 1,5-shifts should be suprafacial, whereas
1,7-shifts should be antarafacial. 214
Sigmatropic migration involving alkyl group shifts can also occur.
R
R R R
1,3-alkyl shift 1,5-alkyl shift
When an alkyl group migrates, there is an additional stereochemical feature to consider.
The shift can occur with retention or inversion at the migrating center. The analysis of
sigmatropic shifts of alkyl groups is illustrated in Figure 10.32. The allowed processes
include the suprafacial 1,3-shift with inversion and the suprafacial 1,5-shift with
retention.
suprafacial 1,3 suprafacial 1,5 antarafacial 1,7
Hückel, 4 electrons Hückel, 6 electrons Mobius, 8 electrons
antiaromatic aromatic aromatic
forbidden allowed allowed
Fig. 10.31. Classification of sigmatropic hydrogen shifts with respect to basis set
orbitals.