Page 924 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 924
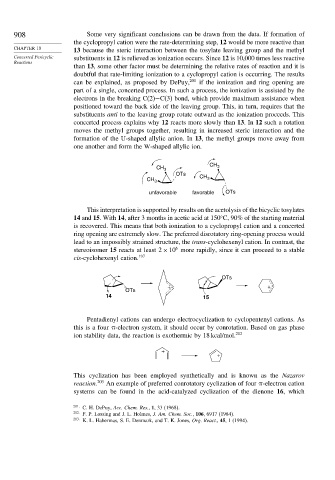
908 Some very significant conclusions can be drawn from the data. If formation of
the cyclopropyl cation were the rate-determining step, 12 would be more reactive than
CHAPTER 10 13 because the steric interaction between the tosylate leaving group and the methyl
Concerted Pericyclic substituents in 12 is relieved as ionization occurs. Since 12 is 10,000 times less reactive
Reactions
than 13, some other factor must be determining the relative rates of reaction and it is
doubtful that rate-limiting ionization to a cyclopropyl cation is occurring. The results
can be explained, as proposed by DePuy, 201 if the ionization and ring opening are
part of a single, concerted process. In such a process, the ionization is assisted by the
electrons in the breaking C(2)−C(3) bond, which provide maximum assistance when
positioned toward the back side of the leaving group. This, in turn, requires that the
substituents anti to the leaving group rotate outward as the ionization proceeds. This
concerted process explains why 12 reacts more slowly than 13.In 12 such a rotation
moves the methyl groups together, resulting in increased steric interaction and the
formation of the U-shaped allylic anion. In 13, the methyl groups move away from
one another and form the W-shaped allylic ion.
CH 3 CH 3
OTs
CH 3 CH 3
unfavorable favorable OTs
This interpretation is supported by results on the acetolysis of the bicyclic tosylates
14 and 15. With 14, after 3 months in acetic acid at 150 C, 90% of the starting material
is recovered. This means that both ionization to a cyclopropyl cation and a concerted
ring opening are extremely slow. The preferred disrotatory ring-opening process would
lead to an impossibly strained structure, the trans-cyclohexenyl cation. In contrast, the
6
stereoisomer 15 reacts at least 2 × 10 more rapidly, since it can proceed to a stable
cis-cyclohexenyl cation. 197
OTs
+ +
OTs
14 15
Pentadienyl cations can undergo electrocyclization to cyclopentenyl cations. As
this is a four -electron system, it should occur by conrotation. Based on gas phase
ion stability data, the reaction is exothermic by 18 kcal/mol. 202
+
+
This cyclization has been employed synthetically and is known as the Nazarov
reaction. 203 An example of preferred conrotatory cyclization of four -electron cation
systems can be found in the acid-catalyzed cyclization of the dienone 16, which
201
C. H. DePuy, Acc. Chem. Res., 1, 33 (1968).
202 F. P. Lossing and J. L. Holmes, J. Am. Chem. Soc., 106, 6917 (1984).
203
K. L. Habermas, S. E. Denmark, and T. K. Jones, Org. React., 45, 1 (1994).