Page 885 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 885
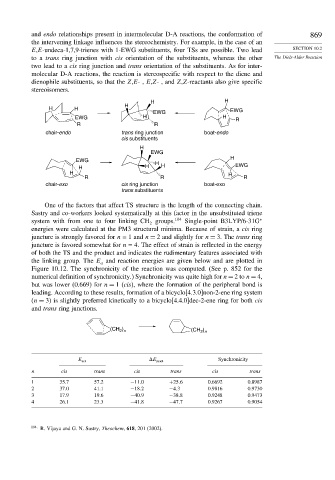
and endo relationships present in intermolecular D-A reactions, the conformation of 869
the intervening linkage influences the stereochemistry. For example, in the case of an
E,E-undeca-1,7,9-trienes with 1-EWG substituents, four TSs are possible. Two lead SECTION 10.2
to a trans ring junction with cis orientation of the substituents, whereas the other The Diels-Alder Reaction
two lead to a cis ring junction and trans orientation of the substituents. As for inter-
molecular D-A reactions, the reaction is stereospecific with respect to the diene and
dienophile substituents, so that the Z,E-, E,Z- , and Z,Z-reactants also give specific
stereoisomers.
H H
H
H H EWG
EWG
EWG H H R
R R
chair-endo trans ring junction boat-endo
cis substituents
H
EWG
EWG H H
H H H EWG
H H
R R R
chair-exo cis ring junction boat-exo
trans substituents
One of the factors that affect TS structure is the length of the connecting chain.
Sastry and co-workers looked systematically at this factor in the unsubstituted triene
system with from one to four linking CH groups. 104 Single-point B3LYP/6-31G ∗
2
energies were calculated at the PM3 structural minima. Because of strain, a cis ring
juncture is strongly favored for n = 1 and n = 2 and slightly for n = 3. The trans ring
juncture is favored somewhat for n = 4. The effect of strain is reflected in the energy
of both the TS and the product and indicates the rudimentary features associated with
the linking group. The E and reaction energies are given below and are plotted in
a
Figure 10.12. The synchronicity of the reaction was computed. (See p. 852 for the
numerical definition of synchronicity.) Synchronicity was quite high for n = 2to n = 4,
but was lower (0.669) for n = 1(cis), where the formation of the peripheral bond is
leading. According to these results, formation of a bicyclo[4.3.0]non-2-ene ring system
(n = 3) is slightly preferred kinetically to a bicyclo[4.4.0]dec-2-ene ring for both cis
and trans ring junctions.
(CH ) (CH )
2 n
2 n
Synchronicity
E act E react
n cis trans cis trans cis trans
1 35.7 57.2 −11 0 +25 6 0.6692 0.8987
2 37.0 41.1 −18 2 −4 3 0.9816 0.9730
3 17.9 19.6 −40 9 −38 8 0.9248 0.9473
4 26.1 23.3 −41 8 −47 7 0.9267 0.9054
104
R. Vijaya and G. N. Sastry, Theochem, 618, 201 (2002).