Page 883 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 883
10.2.6.2. Enantioselective Catalysts for Diels-Alder Reactions Enantioselectivity can 867
94
also be achieved with chiral catalysts. Many of the most efficient catalysts involve a
chiral ligand in conjunction with a metal ion that acts as a Lewis acid. The metal ion SECTION 10.2
provides the electron-attracting capacity and in conjunction with the ligand establishes The Diels-Alder Reaction
a chiral environment at the catalytic site. Several boron compounds are also good
catalysts, with the boron playing the role of the Lewis acid. The ligands typically have
bulky substituents, often substituted aromatic rings. The ligands are usually derived
from readily available chiral substances, such as amino acids. In addition to the Lewis
acid complexation and steric effects, stacking and hydrogen bonding can contribute
to the structure of the catalytic complex. The effectiveness of the catalyst is related to
the proximity of the chiral features to the reaction center. If the chiral environment is
too remote from the catalytic site, it does not control the enantioselectivity effectively.
This proposition was tested for a number of catalysts for the D-A reaction and generally
found to be true. 95
2+ 96
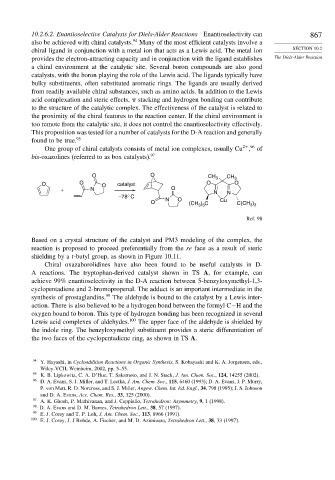
One group of chiral catalysts consists of metal ion complexes, usually Cu , of
bis-oxazolines (referred to as box catalysts). 97
O O CH 3 CH 3
O O O catalyst O O
+ N O
N N
–78° C N O
O (CH ) C Cu C(CH )
3 3
3 3
Ref. 98
Based on a crystal structure of the catalyst and PM3 modeling of the complex, the
reaction is proposed to proceed preferentially from the re face as a result of steric
shielding by a t-butyl group, as shown in Figure 10.11.
Chiral oxazaborolidines have also been found to be useful catalysts in D-
A reactions. The tryptophan-derived catalyst shown in TS A, for example, can
achieve 99% enantioselectivity in the D-A reaction between 5-benzyloxymethyl-1,3-
cyclopentadiene and 2-bromopropenal. The adduct is an important intermediate in the
synthesis of prostaglandins. 99 The aldehyde is bound to the catalyst by a Lewis inter-
action. There is also believed to be a hydrogen bond between the formyl C−H and the
oxygen bound to boron. This type of hydrogen bonding has been recognized in several
Lewis acid complexes of aldehydes. 100 The upper face of the aldehyde is shielded by
the indole ring. The benzyloxymethyl substituent provides a steric differentiation of
the two faces of the cyclopentadiene ring, as shown in TS A.
94 Y. Hayashi, in Cycloaddition Reactions in Organic Synthesis, S. Kobayashi and K. A. Jorgensen, eds.,
Wiley-VCH, Weinheim, 2002, pp. 5–55.
95
K. B. Lipkowitz, C. A. D’Hue, T. Sakamoto, and J. N. Stack, J. Am. Chem. Soc., 124, 14255 (2002).
96 D. A. Evans, S. J. Miller, and T. Lectka, J. Am. Chem. Soc., 115, 6460 (1993); D. A. Evans, J. P. Murry,
P. von Matt, R. D. Norcross, and S. J. Miller, Angew. Chem. Int. Ed. Engl., 34, 798 (1995); J. S. Johnson
and D. A. Evans, Acc. Chem. Res., 33, 325 (2000).
97
A. K. Ghosh, P. Mathivanan, and J. Cappiello, Tetrahedron: Asymmetry, 9, 1 (1998).
98
D. A. Evans and D. M. Barnes, Tetrahedron Lett., 38, 57 (1997).
99 E. J. Corey and T. P. Loh, J. Am. Chem. Soc., 113, 8966 (1991).
100
E. J. Corey, J. J Rohde, A. Fischer, and M. D. Azimioara, Tetrahedron Lett., 38, 33 (1997).