Page 95 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 95
74
CHAPTER 1
Chemical Bonding
and Molecular Structure
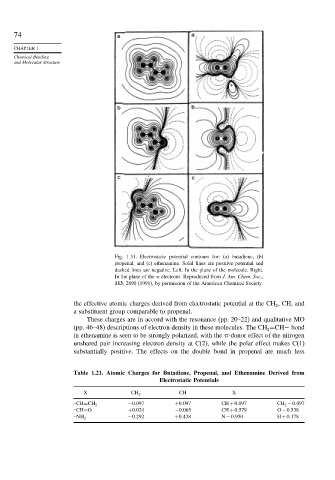
Fig. 1.31. Electrostatic potential contours for: (a) butadiene, (b)
propenal, and (c) ethenamine. Solid lines are positive potential and
dashed lines are negative. Left. In the plane of the molecule. Right.
In the plane of the electrons. Reproduced from J. Am. Chem. Soc.,
113, 2890 (1991), by permission of the American Chemical Society.
the effective atomic charges derived from electrostatic potential at the CH , CH, and
2
a substituent group comparable to propenal.
These charges are in accord with the resonance (pp. 20–22) and qualitative MO
(pp. 46–48) descriptions of electron density in these molecules. The CH =CH− bond
2
in ethenamine is seen to be strongly polarized, with the -donor effect of the nitrogen
unshared pair increasing electron density at C(2), while the polar effect makes C(1)
substantially positive. The effects on the double bond in propenal are much less
Table 1.21. Atomic Charges for Butadiene, Propenal, and Ethenamine Derived from
Electrostatic Potentials
X CH 2 CH X
−0 097 +0 097 CH+0 097 CH 2 −0 097
–CH=CH 2
–CH=O +0 024 −0 065 CH+0 579 O−0 538
−0 292 +0 438 N −0 950 H+0 178
–NH 2