Page 96 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 96
75
SECTION 1.4
Representation of
Electron Density
Distribution
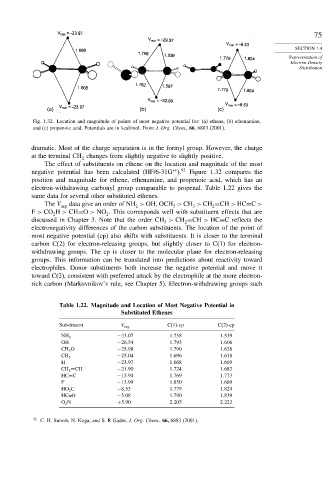
Fig. 1.32. Location and magnitude of points of most negative potential for: (a) ethene, (b) ethenamine,
and (c) propenoic acid. Potentials are in kcal/mol. From J. Org. Chem., 66, 6883 (2001).
dramatic. Most of the charge separation is in the formyl group. However, the charge
at the terminal CH changes from slightly negative to slightly positive.
2
The effect of substituents on ethene on the location and magnitude of the most
∗∗ 92
negative potential has been calculated (HF/6-31G ). Figure 1.32 compares the
position and magnitude for ethene, ethenamine, and propenoic acid, which has an
electron-withdrawing carbonyl group comparable to propenal. Table 1.22 gives the
same data for several other substituted ethenes.
The V neg data give an order of NH > OH, OCH > CH > CH =CH > HC≡C >
3
2
2
3
F > CO H > CH=O > NO . This corresponds well with substituent effects that are
2
2
discussed in Chapter 3. Note that the order CH > CH =CH > HC≡C reflects the
2
3
electronegativity differences of the carbon substituents. The location of the point of
most negative potential (cp) also shifts with substituents. It is closer to the terminal
carbon C(2) for electron-releasing groups, but slightly closer to C(1) for electron-
withdrawing groups. The cp is closer to the molecular plane for electron-releasing
groups. This information can be translated into predictions about reactivity toward
electrophiles. Donor substituents both increase the negative potential and move it
toward C(2), consistent with preferred attack by the electrophile at the more electron-
rich carbon (Markovnikov’s rule, see Chapter 5). Electron-withdrawing groups such
Table 1.22. Magnitude and Location of Most Negative Potential in
Substituted Ethenes
Substituent V neg C(1)-cp C(2)-cp
−33 07 1 758 1 539
NH 2
OH −26 54 1 795 1 606
CH 3 O −25 98 1 790 1 628
−25 04 1 696 1 618
CH 3
H −23 97 1 668 1 669
CH 2 =CH −21 90 1 724 1 682
HC≡C −15 94 1 769 1 773
F −13 99 1 850 1 689
HO 2 C −8 53 1 779 1 824
HC=O −5 08 1 790 1 839
O 2 N +5 90 2 205 2 223
92
C. H. Suresh, N. Koga, and S. R Gadre, J. Org. Chem., 66, 6883 (2001).