Page 93 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 93
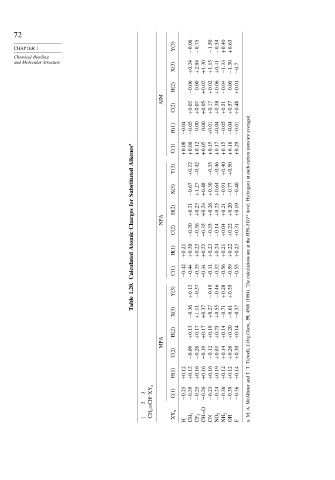
72
Y(3) −0 06 −0 75 −1 50 −0 54 +0 40 +0 63
CHAPTER 1
Chemical Bonding
and Molecular Structure
X(3) +0 24 +2 04 +1 30 +1 15 +0 41 −1 33 −1 30 −0 7
H(2) −0 06 0.00 +0 02 +0 02 +0 06 −0 04 0.00 +0 01
AIM
C(2) +0 02 +0 07 +0 05 +0 17 +0 38 +0 51 +0 57 +0 48
H(1) −0 04 −0 05 0.00 0.00 +0 01 +0 04 −0 05 −0 04 −0 01 averaged.
are
Alkenes a C(1) +0 08 +0 08 +0 22 +0 12 −0 42 +0 05 +0 15 −0 35 +0 17 −0 46 +0 15 +0 40 +0 18 +0 50 +0 29 atom carbon
Substituted Y(3) X(3) −0 67 +1 27 +0 48 +0 30 +0 64 −0 91 −0 77 −0 40 each at Hydrogens
for H(2) +0 21 +0 23 +0 24 +0 26 +0 25 +0 21 +0 20 +0 19 level.
Charges NPA HF6-31G ∗∗
Atomic C(2) H(1) +0 21 −0 20 +0 20 −0 36 +0 23 −0 35 +0 22 −0 35 +0 23 −0 14 +0 24 +0 04 +0 21 +0 22 +0 22 +0 31 +0 23 the at are
Calculated C(1) −0 42 −0 44 −0 35 −0 34 −0 31 −0 32 −0 56 −0 59 −0 55 calculations
1.20. Y(3) +0 12 −0 37 −0 45 −0 46 +0 28 +0 35 The (1994).
Table
X(3) −0 36 +1 11 +0 37 +0 27 +0 55 −0 71 −0 61 −0 37 4506 59,
H(2) +0 13 +0 17 +0 17 +0 19 +0 25 +0 14 +0 20 +0 14 J.Org.Chem.,
MPA
C(2) −0 09 −0 26 −0 19 −0 12 +0 03 +0 14 +0 26 +0 30 Tidwell,
H(1) +0 12 +0 12 +0 16 +0 16 +0 16 +0 19 +0 12 +0 12 +0 14 T. T. and
CH 2 =CH–XY n
3 C(1) −0 25 −0 29 −0 25 −0 26 −0 23 −0 24 −0 36 −0 39 −0 36 McAllister
2 A.
1 XY n CH 3 CF 3 CH=O CN NO 2 NH 2 OH M.
H F a.