Page 89 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 89
68
CHAPTER 1
Chemical Bonding
and Molecular Structure
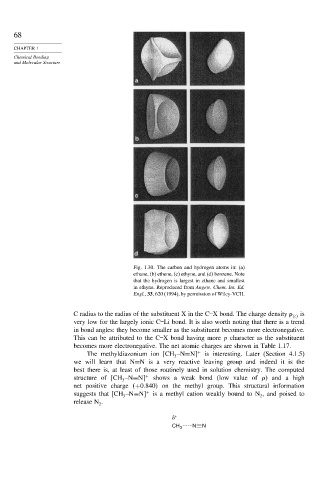
Fig. 1.30. The carbon and hydrogen atoms in: (a)
ethane, (b) ethene, (c) ethyne, and (d) benzene. Note
that the hydrogen is largest in ethane and smallest
in ethyne. Reproduced from Angew. Chem. Int. Ed.
Engl., 33, 620 (1994), by permission of Wiley-VCH.
C radius to the radius of the substituent X in the C–X bond. The charge density c is
very low for the largely ionic C–Li bond. It is also worth noting that there is a trend
in bond angles: they become smaller as the substituent becomes more electronegative.
This can be attributed to the C–X bond having more p character as the substituent
becomes more electronegative. The net atomic charges are shown in Table 1.17.
The methyldiazonium ion CH –N≡N + is interesting. Later (Section 4.1.5)
3
we will learn that N≡N is a very reactive leaving group and indeed it is the
best there is, at least of those routinely used in solution chemistry. The computed
structure of CH –N≡N + shows a weak bond (low value of ") and a high
3
net positive charge +0 840 on the methyl group. This structural information
+
suggests that CH –N≡N is a methyl cation weakly bound to N , and poised to
3
2
release N .
2
δ +
CH 3 N N