Page 87 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 87
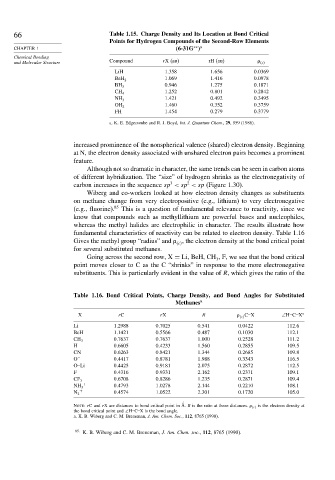
66 Table 1.15. Charge Density and Its Location at Bond Critical
Points for Hydrogen Compounds of the Second-Row Elements
∗∗ a
CHAPTER 1 (6-31G )
Chemical Bonding
and Molecular Structure Compound rX (au) rH (au) c
LiH 1 358 1 656 0 0369
1 069 1 416 0 0978
BeH 2
0 946 1 275 0 1871
BH 3
1 252 0 801 0 2842
CH 4
1 421 0 492 0 3495
NH 3
1 460 0 352 0 3759
OH 2
FH 1 454 0 279 0 3779
a. K. E. Edgecombe and R. J. Boyd, Int. J. Quantum Chem., 29, 959 (1986).
increased prominence of the nonspherical valence (shared) electron density. Beginning
at N, the electron density associated with unshared electron pairs becomes a prominent
feature.
Although not so dramatic in character, the same trends can be seen in carbon atoms
of different hybridization. The “size” of hydrogen shrinks as the electronegativity of
2
3
carbon increases in the sequence sp <sp <sp (Figure 1.30).
Wiberg and co-workers looked at how electron density changes as substituents
on methane change from very electropositive (e.g., lithium) to very electronegative
(e.g., fluorine). 85 This is a question of fundamental relevance to reactivity, since we
know that compounds such as methyllithium are powerful bases and nucleophiles,
whereas the methyl halides are electrophilic in character. The results illustrate how
fundamental characteristics of reactivity can be related to electron density. Table 1.16
Gives the methyl group “radius” and , the electron density at the bond critical point
c
for several substituted methanes.
Going across the second row, X = Li, BeH, CH , F, we see that the bond critical
3
point moves closer to C as the C “shrinks” in response to the more electronegative
substituents. This is particularly evident in the value of R, which gives the ratio of the
Table 1.16. Bond Critical Points, Charge Density, and Bond Angles for Substituted
Methanes a
X rC rX R c C–X ∠H–C–X
Li 1 2988 0 7025 0 541 0 0422 112 6
BeH 1 1421 0 5566 0 487 0 1030 112 1
0 7637 0 7637 1 000 0 2528 111 2
CH 3
H 0 6605 0 4233 1 560 0 2855 109 5
CN 0 6263 0 8421 1 344 0 2685 109 8
O − 0 4417 0 8781 1 988 0 3343 116 5
O–Li 0 4425 0 9181 2 075 0 2872 112 5
F 0 4316 0 9331 2 162 0 2371 109 1
0 6708 0 8286 1 235 0 2871 109 4
CF 3
+ 0 4793 1 0278 2 144 0 2210 108 1
NH 3
+ 0 4574 1 0522 2 301 0 1720 105 0
N 2
´
Note: rC and rX are distances to bond critical point in Å. R is the ratio at these distances. c is the electron density at
the bond critical point and ∠H–C–X is the bond angle.
a. K. B. Wiberg and C. M. Breneman, J. Am. Chem. Soc., 112, 8765 (1990).
85
K. B. Wiberg and C. M. Breneman, J. Am. Chen. soc., 112, 8765 (1990).