Page 83 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 83
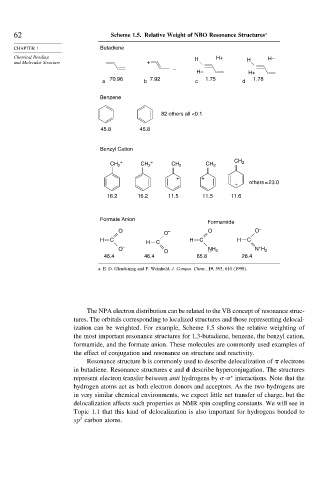
62 Scheme 1.5. Relative Weight of NBO Resonance Structures a
CHAPTER 1 Butadiene
Chemical Bonding H H+ H–
and Molecular Structure + H
–
H– H+
70.96 7.92 1.75 1.78
a b c d
Benzene
82 others all <0.1
45.8 45.8
Benzyl Cation
CH 2 + CH 2 + CH 2 CH 2 CH 2
+ +
+ others = 23.0
16.2 16.2 11.5 11.5 11.6
Formate Anion
Formamide
O – O O –
O
H C H C H C H C
+
O – NH N H
O 2 2
46.4 46.4 65.8 28.4
a. E. D. Glendening and F. Weinhold, J. Comput. Chem., 19, 593, 610 (1998).
The NPA electron distribution can be related to the VB concept of resonance struc-
tures. The orbitals corresponding to localized structures and those representing delocal-
ization can be weighted. For example, Scheme 1.5 shows the relative weighting of
the most important resonance structures for 1,3-butadiene, benzene, the benzyl cation,
formamide, and the formate anion. These molecules are commonly used examples of
the effect of conjugation and resonance on structure and reactivity.
Resonance structure b is commonly used to describe delocalization of electrons
in butadiene. Resonance structures c and d describe hyperconjugation. The structures
∗
represent electron transfer between anti hydrogens by - interactions. Note that the
hydrogen atoms act as both electron donors and acceptors. As the two hydrogens are
in very similar chemical environments, we expect little net transfer of charge, but the
delocalization affects such properties as NMR spin coupling constants. We will see in
Topic 1.1 that this kind of delocalization is also important for hydrogens bonded to
3
sp carbon atoms.