Page 85 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 85
64 electron density accumulates between the two nuclei, relative to electron distribution
in spherical atoms, as indicated earlier in Figure 1.1. Although there is an electron
CHAPTER 1 density minimum on the bond path midway between the two hydrogens, this minimum
Chemical Bonding is a maximum with respect to displacement perpendicular to the bond path.
and Molecular Structure
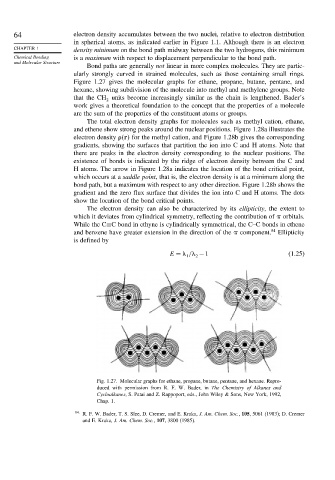
Bond paths are generally not linear in more complex molecules. They are partic-
ularly strongly curved in strained molecules, such as those containing small rings.
Figure 1.27 gives the molecular graphs for ethane, propane, butane, pentane, and
hexane, showing subdivision of the molecule into methyl and methylene groups. Note
that the CH units become increasingly similar as the chain is lengthened. Bader’s
2
work gives a theoretical foundation to the concept that the properties of a molecule
are the sum of the properties of the constituent atoms or groups.
The total electron density graphs for molecules such as methyl cation, ethane,
and ethene show strong peaks around the nuclear positions. Figure 1.28a illustrates the
electron density r for the methyl cation, and Figure 1.28b gives the corresponding
gradients, showing the surfaces that partition the ion into C and H atoms. Note that
there are peaks in the electron density corresponding to the nuclear positions. The
existence of bonds is indicated by the ridge of electron density between the C and
H atoms. The arrow in Figure 1.28a indicates the location of the bond critical point,
which occurs at a saddle point, that is, the electron density is at a minimum along the
bond path, but a maximum with respect to any other direction. Figure 1.28b shows the
gradient and the zero flux surface that divides the ion into C and H atoms. The dots
show the location of the bond critical points.
The electron density can also be characterized by its ellipticity, the extent to
which it deviates from cylindrical symmetry, reflecting the contribution of orbitals.
While the C≡C bond in ethyne is cylindrically symmetrical, the C–C bonds in ethene
and benzene have greater extension in the direction of the component. 84 Ellipticity
is defined by
E = ' /' −1 (1.25)
1
2
Fig. 1.27. Molecular graphs for ethane, propane, butane, pentane, and hexane. Repro-
duced with permission from R. F. W. Bader, in The Chemistry of Alkanes and
Cycloalkanes, S. Patai and Z. Rappoport, eds., John Wiley & Sons, New York, 1992,
Chap. 1.
84
R. F. W. Bader, T. S. Slee, D. Cremer, and E. Kraka, J. Am. Chem. Soc., 105, 5061 (1983); D. Cremer
and E. Kraka, J. Am. Chem. Soc., 107, 3800 (1985).