Page 90 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 90
There is a remarkable difference in the stability of methyl and ethyldiazonium 69
86
ions. The affinity of CH + for N (C–N bond strength) based on thermodynamic data
3 2
is 45±7kcal/mol. Computational results give values of 43 5±1kcal/mol. The ethyl- SECTION 1.4
diazonium ion is much less stable and the computed bond strength is only 11.5 kcal/mol, Representation of
Electron Density
some 32 kcal/mol less that for methyldiazonium ion. Two aspects of this comparison Distribution
are worth remembering for future reference: (1) The very unstable methyldiazonium
ion is the most stable of the alkyl diazonium ions by a considerable margin. Later
(Section 11.2.1, Part B) we will examine aryldiazonium ions and find that they are
substantially more stable. (2) The indication that because it is better able to disperse
the positive charge, an ethyl group binds nitrogen even more weakly than a methyl
group presages the major differences in carbocation stability (methyl < pri < sec <
tert) that we explore in Chapters 3 and 4.
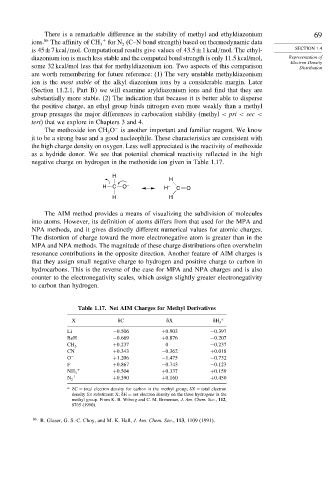
−
The methoxide ion CH O is another important and familiar reagent. We know
3
it to be a strong base and a good nucleophile. These characteristics are consistent with
the high charge density on oxygen. Less well appreciated is the reactivity of methoxide
as a hydride donor. We see that potential chemical reactivity reflected in the high
negative charge on hydrogen in the methoxide ion given in Table 1.17.
H
H
H C O – H – C O
H H
The AIM method provides a means of visualizing the subdivision of molecules
into atoms. However, its definition of atoms differs from that used for the MPA and
NPA methods, and it gives distinctly different numerical values for atomic charges.
The distortion of charge toward the more electronegative atom is greater than in the
MPA and NPA methods. The magnitude of these charge distributions often overwhelm
resonance contributions in the opposite direction. Another feature of AIM charges is
that they assign small negative charge to hydrogen and positive charge to carbon in
hydrocarbons. This is the reverse of the case for MPA and NPA charges and is also
counter to the electronegativity scales, which assign slightly greater electronegativity
to carbon than hydrogen.
Table 1.17. Net AIM Charges for Methyl Derivatives
X &C &X &H 3 a
Li −0 506 +0 903 −0 397
BeH −0 669 +0 876 −0 207
+0 237 0 −0 237
CH 3
CN +0 343 −0 362 +0 018
O − +1 206 −1 475 −0 732
F +0 867 −0 743 −0 123
+ +0 504 +0 337 +0 159
NH 3
+ +0 390 +0 160 +0 450
N 2
a
&C = total electron density for carbon in the methyl group; &X = total electron
density for substituent X; &H = net electron density on the three hydrogens in the
methyl group. From K. B. Wiberg and C. M. Breneman, J. Am. Chem. Soc., 112,
8765 (1990).
86
R. Glaser, G. S.-C. Choy, and M. K. Hall, J. Am. Chem. Soc., 113, 1109 (1991).