Page 1060 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1060
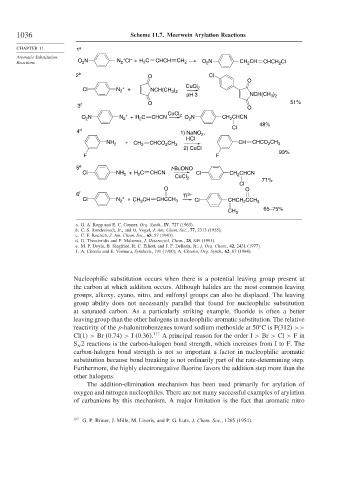
1036 Scheme 11.7. Meerwein Arylation Reactions
CHAPTER 11 1 a
Aromatic Substitution + –
2
2
Reactions O N N Cl + H C CHCH CH 2 O N CH CH CHCH 2 Cl
2
2
2
2 b O Cl
O
+
Cl N + NCH(CH ) CuCl 2
2
3 2
pH 3 NCH(CH )
3 2
3 c O O 51%
CuCl
+
N N + H C CHCN 2 O N CH CHCN
O 2 2 2 2 2
48%
Cl
4 d 1) NaNO ,
2
HCl
NH 2 + CH 2 CHCO CH 3 CH CHCO CH 3
2
2
2) CuCl
93%
F F
5 e t-BuONO
Cl NH + H C CHCN CuCl Cl CH CHCN
2
2
2
2
71%
Cl
O O
6 f Ti 3+
+
Cl N + CH 3 CH CHCCH 3 Cl CHCH CCH 3
2
2
CH 3 65–75%
a. G. A. Ropp and E. C. Coyner, Org. Synth., IV, 727 (1963).
b. C. S. Rondestvedt, Jr., and O. Vogel, J. Am. Chem. Soc., 77, 2313 (1955).
c. C. F. Koelsch, J. Am. Chem. Soc., 65, 57 (1943).
d. G. Theodoridis and P. Malamus, J. Heterocycl. Chem., 28, 849 (1991).
e. M. P. Doyle, B. Siegfried, R. C. Elliott, and J. F. Dellaria, Jr., J. Org. Chem., 42, 2431 (1977).
f. A. Citterio and E. Vismara, Synthesis, 191 (1980); A. Citterio, Org. Synth., 62, 67 (1984).
Nucleophilic substitution occurs when there is a potential leaving group present at
the carbon at which addition occurs. Although halides are the most common leaving
groups, alkoxy, cyano, nitro, and sulfonyl groups can also be displaced. The leaving
group ability does not necessarily parallel that found for nucleophilic substitution
at saturated carbon. As a particularly striking example, fluoride is often a better
leaving group than the other halogens in nucleophilic aromatic substitution. The relative
reactivity of the p-halonitrobenzenes toward sodium methoxide at 50 C is F(312) >>
Cl(1) > Br (0.74) > I (0.36). 117 A principal reason for the order I > Br > Cl > Fin
S 2 reactions is the carbon-halogen bond strength, which increases from I to F. The
N
carbon-halogen bond strength is not so important a factor in nucleophilic aromatic
substitution because bond breaking is not ordinarily part of the rate-determining step.
Furthermore, the highly electronegative fluorine favors the addition step more than the
other halogens.
The addition-elimination mechanism has been used primarily for arylation of
oxygen and nitrogen nucleophiles. There are not many successful examples of arylation
of carbanions by this mechanism. A major limitation is the fact that aromatic nitro
117
G. P. Briner, J. Mille, M. Liveris, and P. G. Lutz, J. Chem. Soc., 1265 (1954).