Page 1063 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1063
Entry 5 involves metallic copper as a catalyst and is probably a metal-catalyzed reaction 1039
(see Section 11.3). The reaction is carried out with excess phenol without solvent.
Entries 6 and 7 are cases of C-arylation, both using 2,4-dinitrochlorobenzene. SECTION 11.2
Nucleophilic Aromatic
Substitution
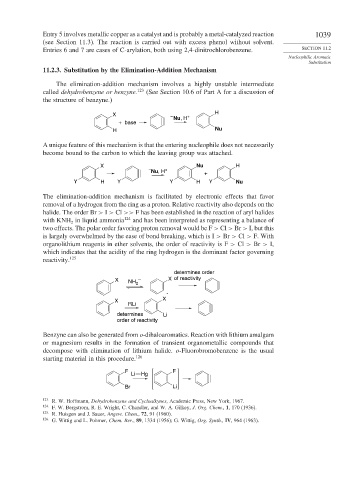
11.2.3. Substitution by the Elimination-Addition Mechanism
The elimination-addition mechanism involves a highly unstable intermediate
called dehydrobenzene or benzyne. 123 (See Section 10.6 of Part A for a discussion of
the structure of benzyne.)
H
X
– +
Nu, H
+ base
H Nu
A unique feature of this mechanism is that the entering nucleophile does not necessarily
become bound to the carbon to which the leaving group was attached.
X Nu H
– Nu, H + +
Y H Y Y H Y Nu
The elimination-addition mechanism is facilitated by electronic effects that favor
removal of a hydrogen from the ring as a proton. Relative reactivity also depends on the
halide. The order Br > I > Cl >> F has been established in the reaction of aryl halides
with KNH in liquid ammonia 124 and has been interpreted as representing a balance of
2
two effects. The polar order favoring proton removal would be F > Cl > Br > I, but this
is largely overwhelmed by the ease of bond breaking, which is I > Br > Cl > F. With
organolithium reagents in ether solvents, the order of reactivity is F > Cl > Br > I,
which indicates that the acidity of the ring hydrogen is the dominant factor governing
reactivity. 125
determines order
X – X of reactivity
NH 2
-
X X
RLi
determines Li
order of reactivity
Benzyne can also be generated from o-dihaloaromatics. Reaction with lithium amalgam
or magnesium results in the formation of transient organometallic compounds that
decompose with elimination of lithium halide. o-Fluorobromobenzene is the usual
starting material in this procedure. 126
F F
Li Hg
Br Li
123
R. W. Hoffmann, Dehydrobenzene and Cycloalkynes, Academic Press, New York, 1967.
124 F. W. Bergstrom, R. E. Wright, C. Chandler, and W. A. Gilkey, J. Org. Chem., 1, 170 (1936).
125 R. Huisgen and J. Sauer, Angew. Chem., 72, 91 (1960).
126
G. Wittig and L. Pohmer, Chem. Ber., 89, 1334 (1956); G. Wittig, Org. Synth., IV, 964 (1963).