Page 1066 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1066
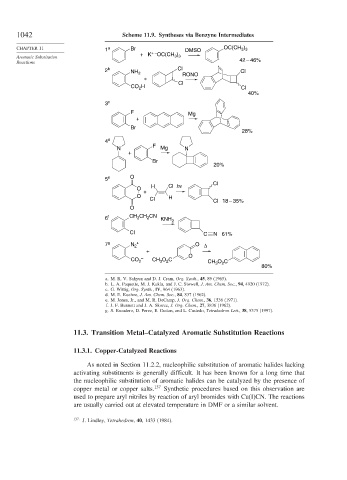
1042 Scheme 11.9. Syntheses via Benzyne Intermediates
CHAPTER 11 1 a Br DMSO OC(CH )
3 3
+ –
+K OC(CH )
Aromatic Substitution 3 3
Reactions 42 – 46%
2 b NH 2 Cl RONO Cl
+
Cl
CO 2 H Cl
40%
3 c
F Mg
+
Br
28%
4 d
F
N Mg N
+
Br
20%
5 e O
H Cl hν Cl
O
+
O H
Cl Cl 18 – 35%
O
6 f CH CH CN KNH 2
2
2
Cl C N 61%
7 g N 2 + O Δ
+
O
CO 2 – CH O C CH O C
3
2
3
2
80%
a. M. R. V. Sahyun and D. J. Cram, Org. Synth., 45, 89 (1965).
b. L. A. Paquette, M. J. Kukla, and J. C. Stowell, J. Am. Chem. Soc., 94, 4920 (1972).
c. G. Wittig, Org. Synth., IV, 964 (1963).
d. M. E. Kuehne, J. Am. Chem. Soc., 84, 837 (1962).
e. M. Jones, Jr., and M. R. DeCamp, J. Org. Chem., 36, 1536 (1971).
f. J. F. Bunnett and J. A. Skorcz, J. Org. Chem., 27, 3836 (1962).
g. S. Escudero, D. Perez, E. Guitan, and L. Castedo, Tetrahedron Lett., 38, 5375 (1997).
11.3. Transition Metal–Catalyzed Aromatic Substitution Reactions
11.3.1. Copper-Catalyzed Reactions
As noted in Section 11.2.2, nucleophilic substitution of aromatic halides lacking
activating substituents is generally difficult. It has been known for a long time that
the nucleophilic substitution of aromatic halides can be catalyzed by the presence of
copper metal or copper salts. 137 Synthetic procedures based on this observation are
used to prepare aryl nitriles by reaction of aryl bromides with Cu(I)CN. The reactions
are usually carried out at elevated temperature in DMF or a similar solvent.
137
J. Lindley, Tetrahedron, 40, 1433 (1984).