Page 1062 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1062
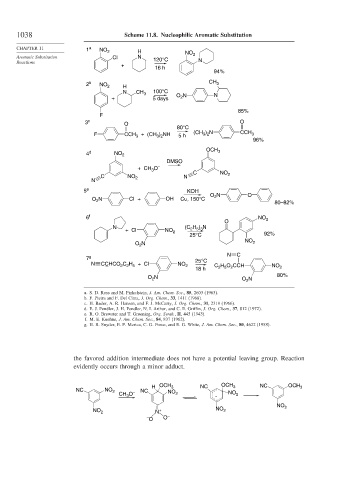
1038 Scheme 11.8. Nucleophilic Aromatic Substitution
CHAPTER 11 1 a NO 2 H
Aromatic Substitution Cl N 120°C NO 2
Reactions N
+ 16 h
94%
2 b CH 3
NO 2
H
N CH 3 100°C N
2
+ 5 days O N
85%
F
3 c O O
80°C
F CCH + (CH ) NH 5 h (CH ) N CCH 3
3 2
3 2
3
96%
OCH
4 d NO 2 3
DMSO
+ CH O –
3
C NO N C NO 2
N 2
5 e KOH
O N O
O N Cl + OH Cu, 150°C 2
2
80–82%
6 f NO 2
O
H ) N
N (C 2 5 3
+ Cl NO 2
25°C 92%
O 2 N NO 2
N C
7 g 25°C
N CCHCO C H + Cl NO
H O CCH
– 2 2 5 2 C 2 5 2 NO 2
18 h
80%
O N O N
2
2
a. S. D. Ross and M. Finkelstein, J. Am. Chem. Soc., 85, 2603 (1963).
b. F. Pietra and F. Del Cima, J. Org. Chem., 33, 1411 (1968).
c. H. Bader, A. R. Hansen, and F. J. McCarty, J. Org. Chem., 31, 2319 (1966).
d. E. J. Fendler, J. H. Fendler, N. I. Arthur, and C. E. Griffin, J. Org. Chem., 37, 812 (1972).
e. R. O. Brewster and T. Groening, Org. Synth., II, 445 (1943).
f. M. E. Kuehne, J. Am. Chem. Soc., 84, 837 (1962).
g. H. R. Snyder, E. P. Merica, C. G. Force, and E. G. White, J. Am. Chem. Soc., 80, 4622 (1958).
the favored addition intermediate does not have a potential leaving group. Reaction
evidently occurs through a minor adduct.
H OCH 3 NC OCH 3 NC OCH 3
NC NO 2 – NC NO
CH O 2 - NO 2
3
NO 2
NO 2 N + NO 2
– O –
O