Page 1065 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1065
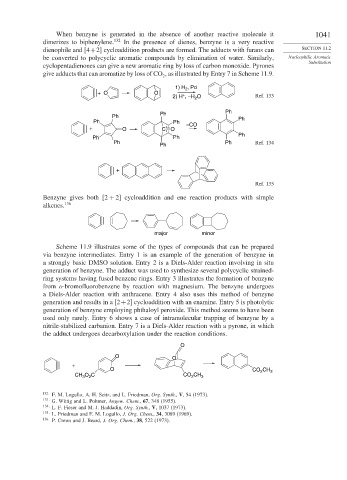
When benzyne is generated in the absence of another reactive molecule it 1041
dimerizes to biphenylene. 132 In the presence of dienes, benzyne is a very reactive
dienophile and [4+2] cycloaddition products are formed. The adducts with furans can SECTION 11.2
be converted to polycyclic aromatic compounds by elimination of water. Similarly, Nucleophilic Aromatic
Substitution
cyclopentadienones can give a new aromatic ring by loss of carbon monoxide. Pyrones
give adducts that can aromatize by loss of CO , as illustrated by Entry 7 in Scheme 11.9.
2
1) H , Pd
2
+ O O
+
2) H , –H O Ref. 133
2
Ph
Ph
Ph
Ph Ph Ph
+ O C O –CO
Ph
Ph Ph
Ph Ph Ref. 134
Ph
+
Ref. 135
Benzyne gives both [2 + 2] cycloaddition and ene reaction products with simple
alkenes. 136
major minor
Scheme 11.9 illustrates some of the types of compounds that can be prepared
via benzyne intermediates. Entry 1 is an example of the generation of benzyne in
a strongly basic DMSO solution. Entry 2 is a Diels-Alder reaction involving in situ
generation of benzyne. The adduct was used to synthesize several polycyclic strained-
ring systems having fused benzene rings. Entry 3 illustrates the formation of benzyne
from o-bromofluorobenzene by reaction with magnesium. The benzyne undergoes
a Diels-Alder reaction with anthracene. Entry 4 also uses this method of benzyne
generation and results in a [2+2] cycloaddition with an enamine. Entry 5 is photolytic
generation of benzyne employing phthaloyl peroxide. This method seems to have been
used only rarely. Entry 6 shows a case of intramolecular trapping of benzyne by a
nitrile-stabilized carbanion. Entry 7 is a Diels-Alder reaction with a pyrone, in which
the adduct undergoes decarboxylation under the reaction conditions.
O
O O
+
O CO CH 3
2
CH O C CO CH 3
2
3
2
132 F. M. Logullo, A. H. Seitz, and L. Friedman, Org. Synth., V, 54 (1973).
133
G. Wittig and L. Pohmer, Angew. Chem., 67, 348 (1955).
134 L. F. Fieser and M. J. Haddadin, Org. Synth., V, 1037 (1973).
135 L. Friedman and F. M. Logullo, J. Org. Chem., 34, 3089 (1969).
136
P. Crews and J. Beard, J. Org. Chem., 38, 522 (1973).