Page 1173 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1173
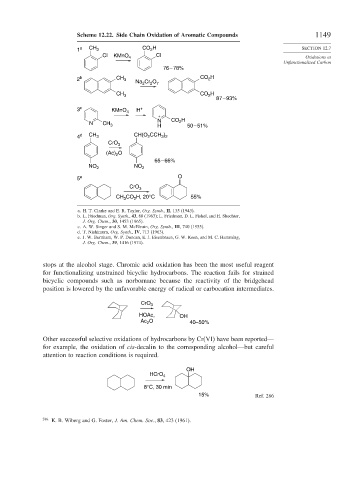
Scheme 12.22. Side Chain Oxidation of Aromatic Compounds 1149
2
1 a CH 3 CO H SECTION 12.7
Cl KMnO 4 Cl Oxidations at
Unfunctionalized Carbon
76 – 78%
2 b CH 3 Na Cr O 7 CO 2 H
2
2
CH 3 CO H
2
87 – 93%
3 c KMnO 4 H +
+
N CO 2 H
N CH 3 H 50 – 51%
3 2
4 d CH 3 CH(O CCH )
2
CrO 3
(Ac) O
2
65 – 66%
NO 2 NO 2
5 e O
CrO 3
CH CO H, 20°C 55%
2
3
a. H. T. Clarke and E. R. Taylor, Org. Synth., II, 135 (1943).
b. L. Friedman, Org. Synth., 43, 80 (1963); L. Friedman, D. L. Fishel, and H. Shechter,
J. Org. Chem., 30, 1453 (1965).
c. A. W. Singer and S. M. McElvain, Org. Synth., III, 740 (1955).
d. T. Nishimura, Org. Synth., IV, 713 (1963).
e. J. W. Burnham, W. P. Duncan, E. J. Eisenbraun, G. W. Keen, and M. C. Hamming,
J. Org. Chem., 39, 1416 (1974).
stops at the alcohol stage. Chromic acid oxidation has been the most useful reagent
for functionalizing unstrained bicyclic hydrocarbons. The reaction fails for strained
bicyclic compounds such as norbornane because the reactivity of the bridgehead
position is lowered by the unfavorable energy of radical or carbocation intermediates.
CrO 3
HOAc, OH
Ac O 40–50%
2
Other successful selective oxidations of hydrocarbons by Cr(VI) have been reported—
for example, the oxidation of cis-decalin to the corresponding alcohol—but careful
attention to reaction conditions is required.
OH
HCrO 4
8°C, 30 min
15% Ref. 286
286
K. B. Wiberg and G. Foster, J. Am. Chem. Soc., 83, 423 (1961).