Page 1293 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1293
TBDMS 1269
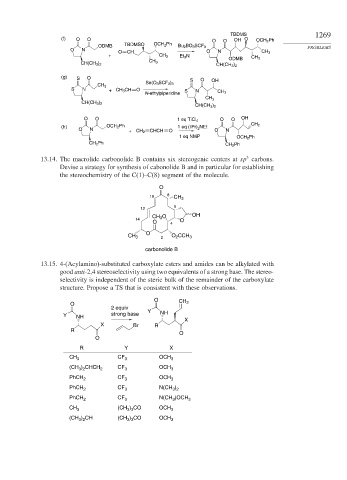
(f) O O O O OH O OCH 2 Ph
ODMB TBDMSO OCH 2 Ph Bu 2 BO 3 SCF 3
O N PROBLEMS
O CH O N CH 3
+ CH 3 Et 3 N
ODMB CH 3
CH 3
CH(CH 3 ) 2
CH(CH 3 ) 2
(g) S O S O OH
Sn(O 3 SCF 3 ) 3
CH 3
S N + CH 3 CH O S N
N-ethylpiperidine CH 3
CH 3
CH(CH 3 ) 2
CH(CH 3 ) 2
O O 1 eq TiCl 4 O O OH
(h) OCH 2 Ph 1 eq (iPr) NEt CH 2
O N 2 O N
+ CH 2 CHCH O
1 eq NMP OCH 2 Ph
CH 2 Ph CH 2 Ph
3
13.14. The macrolide carbonolide B contains six stereogenic centers at sp carbons.
Devise a strategy for synthesis of cabonolide B and in particular for establishing
the stereochemistry of the C(1)–C(8) segment of the molecule.
O
10 8 CH 3
6
12
CH 3 O OH
14 O
O 4
O
CH 3 2 O 2 CCH 3
carbonolide B
13.15. 4-(Acylamino)-substituted carboxylate esters and amides can be alkylated with
good anti-2,4 stereoselectivity using two equivalents of a strong base. The stereo-
selectivity is independent of the steric bulk of the remainder of the carboxylate
structure. Propose a TS that is consistent with these observations.
O
O CH 2
2 equiv
Y NH strong base Y NH
X
X Br R
R
O
O
R Y X
CH 3 CF 3 OCH 3
(CH ) CHCH 2 CF 3 OCH 3
3 2
PhCH 2 CF 3 OCH 3
PhCH 2 CF 3 N(CH )
3 2
PhCH 2 CF 3 N(CH )OCH 3
3
CH 3 (CH ) CO OCH 3
3 3
(CH ) CH (CH ) CO OCH 3
3 3
3 2