Page 1291 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1291
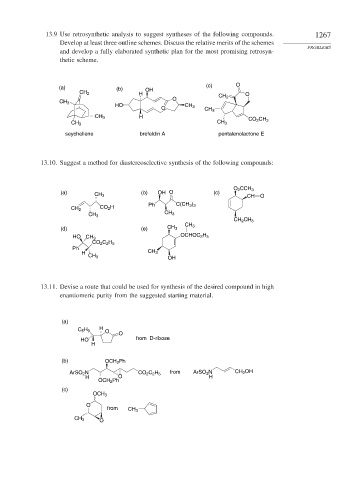
13.9 Use retrosynthetic analysis to suggest syntheses of the following compounds. 1267
Develop at least three outline schemes. Discuss the relative merits of the schemes
PROBLEMS
and develop a fully elaborated synthetic plan for the most promising retrosyn-
thetic scheme.
(a) (b) OH (c) O
CH 2 H O
O CH 2
CH 3
HO CH 3
O CH 3
CH 3 H
CO 2 CH 3
CH 3 CH 3
seychellene brefeldin A pentalenolactone E
13.10. Suggest a method for diastereoselective synthesis of the following compounds:
O 2 CCH 3
(a) (b) OH O (c)
CH 3 CH O
Ph C(CH 3 ) 3
CO 2 H
CH 2
CH 3
CH 3
CH 2 CH 3
CH 3
(d) (e) CH 3
OCHOC 2 H 5
HO CH 3
CO 2 C 2 H 5
Ph
H CH 3
CH 3
OH
13.11. Devise a route that could be used for synthesis of the desired compound in high
enantiomeric purity from the suggested starting material.
(a)
H
C 5 H 9 O O
HO from D-ribose
H
(b) OCH 2 Ph
ArSO 2 N CO 2 C 2 H 5 from ArSO 2 N CH 2 OH
H O H
OCH 2 Ph
(c)
OCH 3
O
from CH 3
CH 3
O