Page 1287 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1287
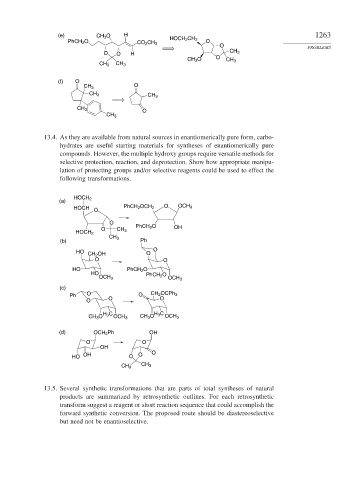
(e) CH 3 O H 1263
HOCH 2 CH 2
PhCH 2 O CO 2 CH 3 O
O PROBLEMS
O O H CH 3
CH 3 O O CH 3
CH 3 CH 3
(f) O
O
CH 3
CH 3
CH 3
CH 3
O
CH 3
13.4. As they are available from natural sources in enantiomerically pure form, carbo-
hydrates are useful starting materials for syntheses of enantiomerically pure
compounds. However, the multiple hydroxy groups require versatile methods for
selective protection, reaction, and deprotection. Show how appropriate manipu-
lation of protecting groups and/or selective reagents could be used to effect the
following transformations.
HOCH 2
(a)
O
HOCH O PhCH 2 OCH 2 OCH 3
O
O CH 3 PhCH 2 O OH
HOCH 2
CH 3
(b) Ph
HO CH 2 OH O O
O O
HO PhCH 2 O
HO PhCH 2 O
OCH 3 OCH 3
(c)
Ph O O O CH 2 OCPh 3
O
O
H 3 C H 3 C
CH 3 O OCH 3 CH 3 O OCH 3
(d) OCH 2 Ph OH
O O
OH
HO OH O O O
CH 3
CH 3
13.5. Several synthetic transformations that are parts of total syntheses of natural
products are summarized by retrosynthetic outlines. For each retrosynthetic
transform suggest a reagent or short reaction sequence that could accomplish the
forward synthetic conversion. The proposed route should be diastereoselective
but need not be enantioselective.