Page 1292 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1292
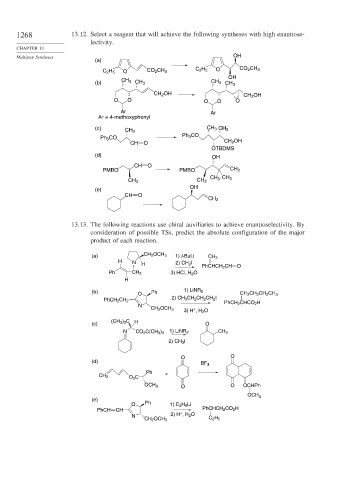
1268 13.12. Select a reagent that will achieve the following syntheses with high enantiose-
lectivity.
CHAPTER 13
Multistep Syntheses OH
(a)
C 2 H 5 O CO 2 CH 3 C 2 H 5 O CO 2 CH 3
OH
(b) CH 3 CH 3 CH 3 CH 3
CH 2 OH CH 2 OH
O O O O O
Ar Ar
Ar = 4-methoxyphenyl
(c) CH 3 CH 3
CH 3
Ph 3 CO
Ph 3 CO
CH O CH 2 OH
OTBDMS
(d)
OH
CH O
PMBO PMBO CH 2
CH 3 CH 3
CH 3 CH 3
OH
(e)
CH O
CH 2
13.13. The following reactions use chiral auxiliaries to achieve enantioselectivity. By
consideration of possible TSs, predict the absolute configuration of the major
product of each reaction.
(a) CH 2 OCH 3 1) t-BuLi CH 3
H N H 2) CH 3 I PhCHCH 2 CH O
Ph CH 2 3) HCl, H 2 O
H
(b) Ph 1) LiNR 2
O CH 2 CH 2 CH 2 CH 3
2) CH 3 CH 2 CH 2 CH 2 I
PhCH 2 CH 2 PhCH 2 CHCO 2 H
N
CH 2 OCH 3 +
3) H , H 2 O
(CH 3 ) 3 C H
(c) O
N CO 2 C(CH 3 ) 3 1) LiNR 2 CH 3
2) CH 3 I
O O
(d)
BF 3
Ph +
CH 2 O 2 C
OCH 3 O O OCHPh
OCH 3
(e)
O Ph 1) C 2 H 5 Li
PhCH CH + PhCHCH 2 CO 2 H
N 2) H , H 2 O
CH 2 OCH 3 C 2 H 5