Page 1288 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1288
1264 (a) O O
CH O
CHAPTER 13 CH 3 CH(CH 3 ) 2
Multistep Syntheses
O
CH 3
CH 3 CH 3
(b) H H O
CH 3 O O O CH 3
O
H 3 C
OH
H 3 C
CH 3 HO
CH 3 OH CH 3 OH
(c) H
HO O O I
CH 2 CN
O
CN
CN N
N NH N N CO 2 CH 3
CO 2 CH 3
O
(d) H
HO H OH
HO H
CH 2 CO 2 C 2 H 5
O
N
N NH CO 2 C 2 H 5 CO 2 C 2 H 5 CN
N N
O CN CN
(e) CH 3
CH 3 CH 3 CO 2 H
CH CHCO 2 C 2 H 5
H
CH 3 O H OH CH 3
O CH 3 CH 3
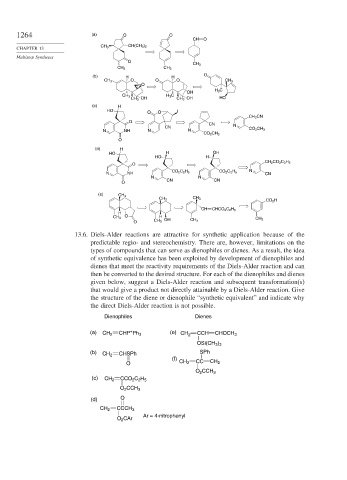
13.6. Diels-Alder reactions are attractive for synthetic application because of the
predictable regio- and stereochemistry. There are, however, limitations on the
types of compounds that can serve as dienophiles or dienes. As a result, the idea
of synthetic equivalence has been exploited by development of dienophiles and
dienes that meet the reactivity requirements of the Diels-Alder reaction and can
then be converted to the desired structure. For each of the dienophiles and dienes
given below, suggest a Diels-Alder reaction and subsequent transformation(s)
that would give a product not directly attainable by a Diels-Alder reaction. Give
the structure of the diene or dienophile “synthetic equivalent” and indicate why
the direct Diels-Alder reaction is not possible.
Dienophiles Dienes
(a) CH 2 CHP Ph 3 (e) CH 2 CCH CHOCH 3
+
OSi(CH 3 ) 3
(b) CHSPh SPh
CH 2
(f)
O CH 2 CC CH 2
O 2 CCH 3
(c) CH 2 CCO 2 C 2 H 5
O 2 CCH 3
(d) O
CH 2 CCCH 3
Ar = 4-nitrophenyl
O 2 CAr