Page 171 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 171
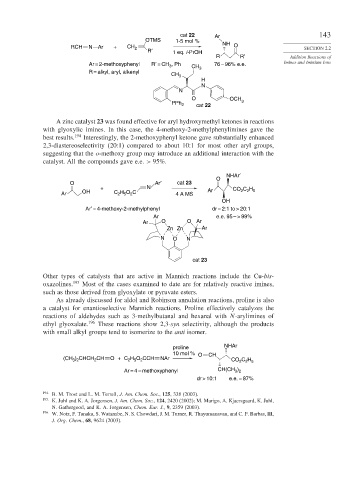
cat 22 Ar 143
OTMS 1-5 mol %
RCH N Ar + CH 2 R′ NH O SECTION 2.2
1 eq. i-PrOH
R R′ Addition Reactions of
Ar = 2-methoxyphenyl R′ = CH , Ph CH 3 76 – 96% e.e. Imines and Iminium Ions
3
R = alkyl, aryl, alkenyl CH
3
H
N
N
O OCH
PPh 2 cat 22 3
A zinc catalyst 23 was found effective for aryl hydroxymethyl ketones in reactions
with glyoxylic imines. In this case, the 4-methoxy-2-methylphenylimines gave the
best results. 194 Interestingly, the 2-methoxyphenyl ketone gave substantially enhanced
2,3-diastereoselectivity (20:1) compared to about 10:1 for most other aryl groups,
suggesting that the o-methoxy group may introduce an additional interaction with the
catalyst. All the compounds gave e.e. > 95%.
NHAr′
O
O Ar′ cat 23
+ N Ar CO C H
H O C
Ar OH C 2 5 2 4 A MS 2 2 5
OH
Ar′ = 4-methoxy-2-methylphenyl dr = 2:1 to > 20:1
Ar e.e. 95 – > 99%
Ar O O Ar
Zn Zn Ar
N O N
cat 23
Other types of catalysts that are active in Mannich reactions include the Cu-bis-
oxazolines. 195 Most of the cases examined to date are for relatively reactive imines,
such as those derived from glyoxylate or pyruvate esters.
As already discussed for aldol and Robinson annulation reactions, proline is also
a catalyst for enantioselective Mannich reactions. Proline effectively catalyzes the
reactions of aldehydes such as 3-methylbutanal and hexanal with N-arylimines of
ethyl glyoxalate. 196 These reactions show 2,3-syn selectivity, although the products
with small alkyl groups tend to isomerize to the anti isomer.
proline NHAr
10 mol % O CH
) CHCH CH O + C H O CCH NAr
(CH 3 2 2 2 5 2 CO C H
2 2 5
)
Ar = 4 – methoxyphenyl CH(CH 3 2
dr > 10:1 e.e. = 87%
194 B. M. Trost and L. M. Terrell, J. Am. Chem. Soc., 125, 338 (2003).
195 K. Juhl and K. A. Jorgensen, J. Am. Chem. Soc., 124, 2420 (2002); M. Marigo, A. Kjaersgaard, K. Juhl,
N. Gathergood, and K. A. Jorgensen, Chem. Eur. J., 9, 2359 (2003).
196
W. Notz, F. Tanaka, S. Watanabe, N. S. Chowdari, J. M. Turner, R. Thayumanavan, and C. F. Barbas, III,
J. Org. Chem., 68, 9624 (2003).