Page 174 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 174
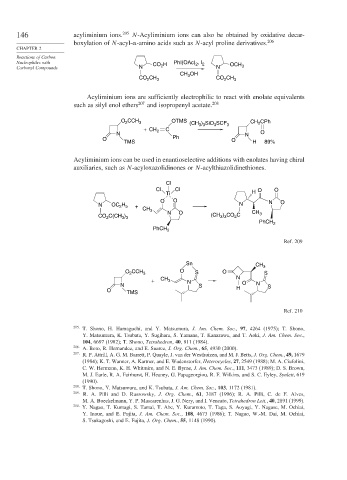
146 acyliminium ions. 205 N-Acyliminium ions can also be obtained by oxidative decar-
boxylation of N-acyl- -amino acids such as N-acyl proline derivatives. 206
CHAPTER 2
Reactions of Carbon
Nucleophiles with H PhI(OAc) , I 2
2
Carbonyl Compounds N CO 2 N OCH 3
CH OH
CH 3 CO CH
CO 2 3 2 3
Acyliminium ions are sufficiently electrophilic to react with enolate equivalents
such as silyl enol ethers 207 and isopropenyl acetate. 208
O CCH 3 OTMS (CH ) SiO SCF 3 CH CPh
2
2
3 3
3
+ CH C
N 2 N O
O Ph O
TMS H 89%
Acyliminium ions can be used in enantioselective additions with enolates having chiral
auxiliaries, such as N-acyloxazolidinones or N-acylthiazolidinethiones.
Cl
Cl Cl H O O
Ti
O O N O
N OC 2 5 + N
H
CH 3
CO C(CH ) N O (CH ) CO C CH 3
3 3
2
3 3
2
PhCH 2
PhCH 2
Ref. 209
Sn CH 3
CCH O O
O 2 3 S S
+ CH 3 N N
N S O N S
O TMS H
Ref. 210
205 T. Shono, H. Hamaguchi, and Y. Matsumura, J. Am. Chem. Soc., 97, 4264 (1975); T. Shono,
Y. Matsumura, K. Tsubata, Y. Sugihara, S. Yamane, T. Kanazawa, and T. Aoki, J. Am. Chem. Soc.,
104, 6697 (1982); T. Shono, Tetrahedron, 40, 811 (1984).
206
A. Boto, R. Hernandez, and E. Suarez, J. Org. Chem., 65, 4930 (2000).
207 R. P. Attrill, A. G. M. Barrett, P. Quayle, J. van der Westhuizen, and M. J. Betts, J. Org. Chem., 49, 1679
(1984); K. T. Wanner, A. Kartner, and E. Wadenstorfer, Heterocycles, 27, 2549 (1988); M. A. Ciufolini,
C. W. Hermann, K. H. Whitmire, and N. E. Byrne, J. Am. Chem. Soc., 111, 3473 (1989); D. S. Brown,
M. J. Earle, R. A. Fairhurst, H. Heaney, G. Papageorgiou, R. F. Wilkins, and S. C. Eyley, Synlett, 619
(1990).
208
T. Shono, Y. Matsumura, and K. Tsubata, J. Am. Chem. Soc., 103, 1172 (1981).
209 R. A. Pilli and D. Russowsky, J. Org. Chem., 61, 3187 (1996); R. A. Pilli, C. de F. Alves,
M. A. Boeckelmann, Y. P. Mascarenhas, J. G. Nery, and I. Vencato, Tetrahedron Lett., 40, 2891 (1999).
210
Y. Nagao, T. Kumagi, S. Tamai, T. Abe, Y. Kuramoto, T. Taga, S. Aoyagi, Y. Nagase, M. Ochiai,
Y. Inoue, and E. Fujita, J. Am. Chem. Soc., 108, 4673 (1986); T. Nagao, W.-M. Dai, M. Ochiai,
S. Tsukagoshi, and E. Fujita, J. Org. Chem., 55, 1148 (1990).