Page 169 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 169
Scheme 2.12. Synthesis and Utilization of Mannich Bases 141
A. Aminomethylation Using the Mannich Reaction SECTION 2.2
+ H Addition Reactions of
1 a PhCOCH 3 + CH O + (CH ) NH Cl – PhCOCH CH N(CH ) Cl – Imines and Iminium Ions
3 2
2
2
2
2
+ 3 2 70%
+ H
b
2 CH COCH 3 + CH 2 O + (CH CH ) NH Cl – CH 3 COCH 2 CH 2 N(C 2 H 5 ) 2 Cl –
2 2
2
3
3
+
66–75%
CF CO H
3
2
c
3 (CH ) CHCOCH + [(CH ) N] CH 2 (CH 3 2 2 2 3 2
) CHCOCH CH N(CH )
3 2
2
3 2
3
4 d OSiMe 3 OLi + O
1) (CH ) N CH
CH Li 3 2 2 CH 2 N(CH )
3 2
3
+ –
THF 2) H O, H , OH
2
87%
5 e O OK O
KH +
(CH ) N CH 2 CH N(CH )
3 2
2
3 2
THF, 0°C I –
88%
B. Reactions Involving Secondary Transformations of Aminomethylation Products.
6 f + 1) 60°C, 6 h CH 2 CCH O
CH 3 CH 2 CH 2 CH O + CH 2 O + (CH ) NH Cl –
3 2 2 2) distill
CH CH 3
2
73%
CH 3
O
7 g O
PhNH 2
+ (CH O) n + CH 2
2
–
CF CO , THF 90%
3
2
8 h O O
NaOH CH 2 CH COPh
2
+ PhCOCH CH N(CH )
3 2
2
2
52%
i
9 PhCOCH CH N(CH ) + KCN PhCOCH CH CN
2
2
2
3 2
2
67%
a. C. E. Maxwell, Org. Synth., III, 305 (1955).
b. A. L. Wilds, R. M. Novak, and K. E. McCaleb, Org. Synth., IV, 281 (1963).
c. M. Gaudry, Y. Jasor, and T. B. Khac, Org. Synth., 59, 153 (1979).
d. S. Danishefsky, T. Kitahara, R. McKee, and P. F. Schuda, J. Am. Chem. Soc., 98, 6715 (1976).
e. J. L. Roberts, P. S. Borromeo, and C. D. Poulter, Tetrahedron Lett., 1621 (1977).
f. C. S. Marvel, R. L. Myers, and J. H. Saunders, J. Am. Chem. Soc., 70, 1694 (1948).
g. J. L. Gras, Tetrahedron Lett., 2111, 2955 (1978).
h. A. C. Cope and E. C. Hermann, J. Am. Chem. Soc., 72, 3405 (1950).
i. E. B. Knott, J. Chem. Soc., 1190 (1947).
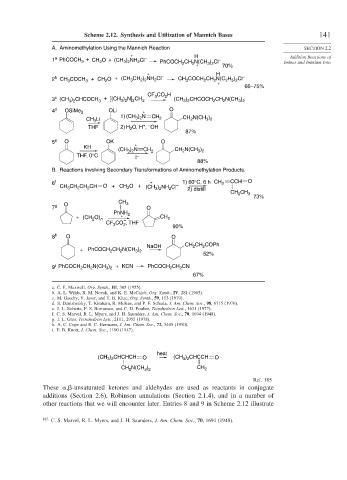
heat
(CH ) CHCHCH O (CH ) CHCCH O
3 2
3 2
N(CH ) CH
CH 2 3 2 2
Ref. 185
These , -unsaturated ketones and aldehydes are used as reactants in conjugate
additions (Section 2.6), Robinson annulations (Section 2.1.4), and in a number of
other reactions that we will encounter later. Entries 8 and 9 in Scheme 2.12 illustrate
185
C. S. Marvel, R. L. Myers, and J. H. Saunders, J. Am. Chem. Soc., 70, 1694 (1948).