Page 173 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 173
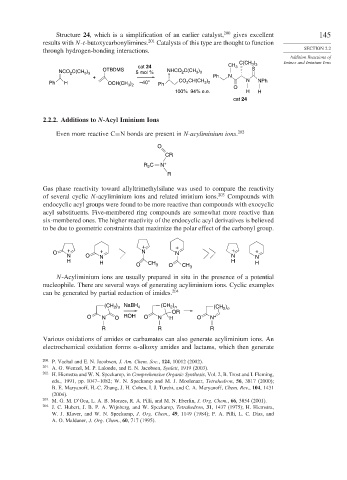
Structure 24, which is a simplification of an earlier catalyst, 200 gives excellent 145
results with N-t-butoxycarbonylimines. 201 Catalysts of this type are thought to function
through hydrogen-bonding interactions. SECTION 2.2
Addition Reactions of
C(CH ) Imines and Iminium Ions
cat 24 CH 3 3 3
NCO C(CH ) OTBDMS 5 mol % NHCO C(CH ) S
2
3 3
3 3
2
+ Ph N
Ph H OCH(CH ) –40° Ph CO 2 CH(CH ) N NPh
3 2
3 2
O
100% 94% e.e. H H
cat 24
2.2.2. Additions to N-Acyl Iminium Ions
Even more reactive C=N bonds are present in N-acyliminium ions. 202
O
CR
R C N +
2
R
Gas phase reactivity toward allyltrimethylsilane was used to compare the reactivity
of several cyclic N-acyliminium ions and related iminium ions. 203 Compounds with
endocyclic acyl groups were found to be more reactive than compounds with exocyclic
acyl substituents. Five-membered ring compounds are somewhat more reactive than
six-membered ones. The higher reactivity of the endocyclic acyl derivatives is believed
to be due to geometric constraints that maximize the polar effect of the carbonyl group.
+ +
+ + + +
O N N
N O N N N
H H O CH 3 O CH 3 H H
N-Acyliminium ions are usually prepared in situ in the presence of a potential
nucleophile. There are several ways of generating acyliminium ions. Cyclic examples
can be generated by partial reduction of imides. 204
) NaBH )
(CH 2 n 4 (CH 2 n (CH 2 ) n
OR
O N O ROH O N H O N +
R R R
Various oxidations of amides or carbamates can also generate acyliminium ions. An
electrochemical oxidation forms -alkoxy amides and lactams, which then generate
200
P. Vachal and E. N. Jacobsen, J. Am. Chem. Soc., 124, 10012 (2002).
201 A. G. Wenzel, M. P. Lalonde, and E. N. Jacobsen, Synlett, 1919 (2003).
202
H. Hiemstra and W. N. Speckamp, in Comprehensive Organic Synthesis, Vol. 2, B. Trost and I. Fleming,
eds., 1991, pp. 1047–1082; W. N. Speckamp and M. J. Moolenaar, Tetrahedron, 56, 3817 (2000);
B. E. Maryanoff, H.-C. Zhang, J. H. Cohen, I. J. Turchi, and C. A. Maryanoff, Chem. Rev., 104, 1431
(2004).
203 M. G. M. D’Oca, L. A. B. Moraes, R. A. Pilli, and M. N. Eberlin, J. Org. Chem., 66, 3854 (2001).
204
J. C. Hubert, J. B. P. A. Wijnberg, and W. Speckamp, Tetrahedron, 31, 1437 (1975); H. Hiemstra,
W. J. Klaver, and W. N. Speckamp, J. Org. Chem., 49, 1149 (1984); P. A. Pilli, L. C. Dias, and
A. O. Maldaner, J. Org. Chem., 60, 717 (1995).