Page 179 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 179
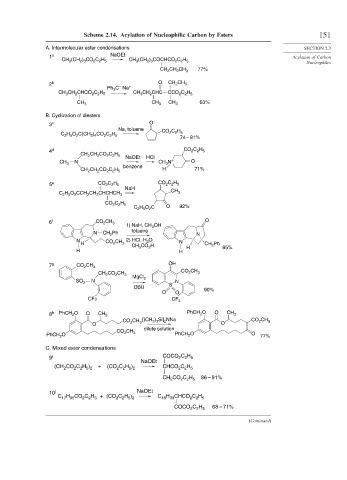
Scheme 2.14. Acylation of Nucleophilic Carbon by Esters 151
A. Intermolecular ester condensations SECTION 2.3
1 a NaOEt Acylation of Carbon
CH 3 (CH 2 ) 3 CO 2 C 2 H 5 CH 3 (CH 2 ) 3 COCHCO 2 C 2 H 5
Nucleophiles
77%
CH 2 CH 2 CH 3
2 b O CH 2 CH 3
–
Ph 3 C Na +
CH 3 CH 2 CHC
CH 3 CH 2 CHCO 2 C 2 H 5 CCO 2 C 2 H 5
63%
CH 3 CH 3 CH 3
B. Cyclization of diesters
3 c O
Na, toluene
CO 2 C 2 H 5
C 2 H 5 O 2 C(CH 2 ) 4 CO 2 C 2 H 5
74 – 81%
4 d CO 2 C 2 H 5
CH 2 CH 2 CO 2 C 2 H 5
NaOEt HCl
N CH 3 N + O
CH 3
benzene
CH 2 CH 2 CO 2 C 2 H 5 H 71%
5 e CO 2 C 2 H 5 CO 2 C 2 H 5
NaH
CH 3
C 2 H 5 O 2 CCH 2 CH 2 CHCHCH 3
CO 2 C 2 H 5
C 2 H 5 O 2 C O 92%
6 f CO CH 3 O
2
1) NaH, CH 3 OH
N CH Ph toluene N
2
N 2) HCl, H 2 O N
H CO 2 CH 3 CH Ph
CH 3 CO H H 2 85%
2
H H
7 g CO CH 3 OH
2
CO CH
CH 2 CO CH 2 3
3
2
MgCl 2
SO 2 N N
DBU S 90%
O O
CF3 CF 3
8 h PhCH O O CH 3 PhCH O O CH 3
2
2
CH [(CH ) Si] NNa CH
CO 2 3 3 3 2 CO 2 3
O O
dilute solution
CO 2 CH 3
PhCH O PhCH O O 77%
2
2
C. Mixed ester condensations
2 2 5
9 i COCO C H
NaOEt
+
(CH 2 CO 2 C 2 H 5 ) 2 (CO 2 C 2 H 5 ) 2 CHCO 2 C 2 H 5
CH CO C H 86 – 91%
2 2 5
2
10 j NaOEt
C H )
C H CO C H + (CO 2 2 5 2 C H CHCO C H
17 35
2 2 5
16 33
2 2 5
COCO C H 68 – 71%
2 2 5
(Continued)