Page 181 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 181
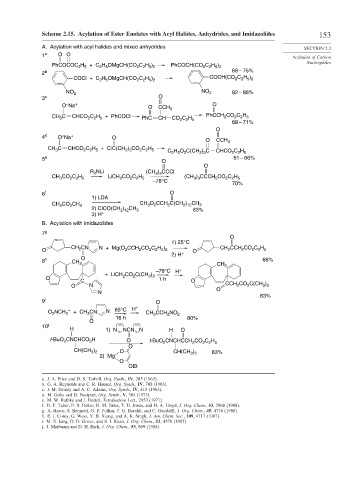
Scheme 2.15. Acylation of Ester Enolates with Acyl Halides, Anhydrides, and Imidazolides 153
A. Acylation with acyl halides and mixed anhydrides SECTION 2.3
1 a O O Acylation of Carbon
Nucleophiles
H OMgCH(CO C H ) C H )
PhCOCOC H + C 2 5 2 2 5 2 PhCOCH(CO 2 2 5 2
2 5
2 b 68 – 75%
COCl + C H OMgCH(CO C H ) COCH(CO C H )
2 2 5 2
2 2 5 2
2 5
NO 2 NO 2 82 – 88%
3 c O
–
O Na + O CCH 3 O
CH C CHCO C H + PhCOCl PhC CH CO C H PhCCH CO C H
2 2 5
2
2 2 5
3
2 2 5
68 – 71%
O
–
4 d O Na + O
O CCH 3
CH C CHCO C H + ClC(CH ) CO C H C H O C(CH ) C CHCO C H
2 2 5
3
2 2 5
2 3
2 3
2 2 5
2
2 5
5 e O 61 – 66%
O
R NLi (CH ) CCCl
3 3
2
CH CO C H LiCH CO C H (CH ) CCCH CO C H
2
2 2 5
3 3
2
2 2 5
2 2 5
3
–78°C
70%
6 f O
1) LDA
CH CO CH 3 CH O CCH C(CH ) CH 3
2
2 12
3
2
3
2
2) ClCO(CH ) CH 3 83%
2 12
+
3) H
B. Acylation with imidazolides
7 g
O
1) 25°C
CH 2 CN N + Mg(O CCH CO C H ) CH CCH CO C H
O 2 2 2 2 5 2 O 2 2 2 2 5
2) H +
8 h CH O 66%
3
CH 3
–78°C H +
+ LiCH CO C(CH )
O 2 2 3 3 1 h
C O
2
3 3
2
O N CCH CO C(CH )
N O
83%
9 i O
O NCH 2 – + CH CN N 65°C H + CH CCH NO 2
3
2
2
3
16 h 80%
O
10 j
H 1) N NCN N H O
t-BuO CNCHCO H O t-BuO CNCHCCH CO C H
2
2
2
2
2 2 5
O
CH(CH ) O CH(CH ) 83%
3 2
2) Mg 3 2
O
OEt
a. J. A. Price and D. S. Tarbell, Org. Synth., IV, 285 (1963).
b. G. A. Reynolds and C. R. Hauser, Org. Synth., IV, 708 (1963).
c. J. M. Straley and A. C. Adams, Org. Synth., IV, 415 (1963).
d. M. Guha and D. Nasipuri, Org. Synth., V, 384 (1973).
e. M. W. Rathke and J. Deitch, Tetrahedron Lett., 2953 (1971).
f. D. F. Taber, P. B. Deker, H. M. Fales, T. H. Jones, and H. A. Lloyd, J. Org. Chem., 53, 2968 (1988).
g. A. Barco, S. Bennetti, G. P. Pollini, P. G. Baraldi, and C. Gandolfi, J. Org. Chem., 45, 4776 (1980).
h. E. J. Corey, G. Wess, Y. B. Xiang, and A. K. Singh, J. Am. Chem. Soc., 109, 4717 (1987).
i. M. E. Jung, D. D. Grove, and S. I. Khan, J. Org. Chem., 52, 4570 (1987).
j. J. Maibaum and D. H. Rich, J. Org. Chem., 53, 869 (1988).