Page 189 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 189
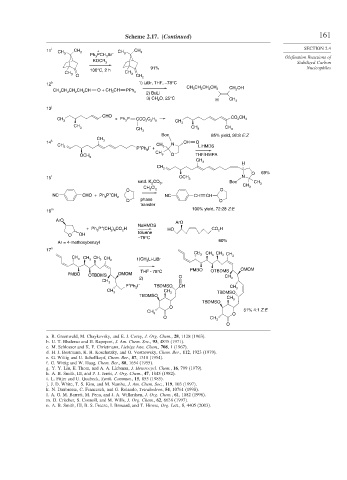
Scheme 2.17. (Continued) 161
11 i CH CH + CH 3 CH 3 SECTION 2.4
3 3 Ph PCH Br –
3 3 Olefination Reactions of
KOCR
3 Stabilized Carbon
91% Nucleophiles
100°C, 2 h
CH CH
3 3
O CH 2
b 1) LiBr, THF, –78°C
12
CH CH CH
CH 3 CH OH
CH CH CH CH CH O + CH CH PPh 2 2 2 2
3 2 2 2 3 3
2) BuLi
O, 25°C
3) CH 2 H CH 3
j
13
CHO CO CH
CH + Ph 3 P CCO 2 C H 2 3
3 2 5 CH 3
CH CH CH
3 CH 3 3
3
Boc 85% yield, 92:8 E:Z
CH
14 k 3 CH N CH O
CH 2 + – 3 LiHMDS
P Ph I +
3
CH
OCH 3 O THF/HMPA
3
CH
3 H
CH
2
O 69%
15 l OCH 3 N
satd. K CO , Boc CH
2 3 CH 3
Cl 3
CH 2
O 2 O
+
NC CHO + Ph P CH NC CH CH
3 2
O phase O
transfer
m 100% yield, 72:28 Z:E
16
ArO ArO
+
+ Ph P (CH ) CO H NaHMDS HO CO H
3 2 4 2 toluene 2
O OH
–78°C
Ar = 4-methoxybenzyl 60%
17 n
CH 3 CH CH CH
CH 3 CH CH CH 1)CH Li-LiBr 3 3 3
3
3
3
3
PMBO OMOM
THF - 78°C OTBDMS
PMBO OTBDMS OMOM O CH
CH 2) 3
3
+
P Ph I – TBDMSO CH CH
CH 3 CH 3
3 3 TBDMSO
TBDMSO CH
TBDMSO 3
O
CH 51% 4:1 Z:E
3 O
O CH
3
O
a. R. Greenwald, M. Chaykovsky, and E. J. Corey, J. Org. Chem., 28, 1128 (1963).
b. U. T. Bhalerao and H. Rapoport, J. Am. Chem. Soc., 93, 4835 (1971).
c. M. Schlosser and K. F. Christmann, Liebigs Ann. Chem., 708, 1 (1967).
d. H. J. Bestmann, K. H. Koschatzky, and O. Vostrowsky, Chem. Ber., 112, 1923 (1979).
e. G. Wittig and U. Schollkopf, Chem. Ber., 87, 1318 (1954).
f. G. Wittig and W. Haag, Chem. Ber., 88, 1654 (1955).
g. Y. Y. Liu, E. Thom, and A. A. Liebman, J. Heterocycl. Chem., 16, 799 (1979).
h. A. B. Smith, III, and P. J. Jerris, J. Org. Chem., 47, 1845 (1982).
i. L. Fitjer and U. Quabeck, Synth. Commun., 15, 855 (1985).
j. J. D. White, T. S. Kim, and M. Nambu, J. Am. Chem. Soc., 119, 103 (1997).
k. N. Daubresse, C. Francesch, and G. Rolando, Tetrahedron, 54, 10761 (1998).
l. A. G. M. Barrett, M. Pena, and J. A. Willardsen, J. Org. Chem., 61, 1082 (1996).
m. D. Critcher, S. Connoll, and M. Wills, J. Org. Chem., 62, 6638 (1997).
n. A. B. Smith, III, B. S. Freeze, I. Brouard, and T. Hirose, Org. Lett., 5, 4405 (2003).