Page 193 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 193
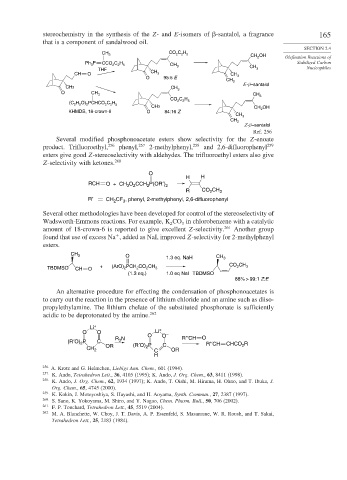
stereochemistry in the synthesis of the Z- and E-isomers of -santalol, a fragrance 165
that is a component of sandalwood oil.
SECTION 2.4
CH 3 CO C H 5 CH OH
2
2
2 Olefination Reactions of
P Stabilized Carbon
Ph 3 CCO 2 C 2 H 5 CH 3 CH
THF 3 Nucleophiles
CH O CH 3 CH
O 95:5 E 3
CH 2
E-β –santalol
CH3 CH 3
O CH
3 CH 3
CO C H
H O) PCHCO C H 2 2 5
5 2 2 2 5
(C 2
CH3 CH OH
KHMDS, 18-crown-6 O 84:16 Z 2
CH 3
CH 2
Z-β –santalol
Ref. 256
Several modified phosphonoacetate esters show selectivity for the Z-enoate
product. Trifluoroethyl, 256 phenyl, 257 2-methylphenyl, 258 and 2,6-difluorophenyl 259
esters give good Z-stereoselectivity with aldehydes. The trifluoroethyl esters also give
Z-selectivity with ketones. 260
O
H H
RCH O + CH O CCH P(OR′) 2
3
2
2
R CO CH 3
2
R′ CH 2 CF , phenyl, 2-methylphenyl, 2,6-difluorophenyl
3
Several other methodologies have been developed for control of the stereoselectivity of
Wadsworth-Emmons reactions. For example, K CO in chlorobenzene with a catalytic
3
2
amount of 18-crown-6 is reported to give excellent Z-selectivity. 261 Another group
+
found that use of excess Na , added as NaI, improved Z-selectivity for 2-methylphenyl
esters.
CH 3 O 1.3 eq. NaH CH 3
TBDMSO CH O + (ArO) PCH CO CH 3 CO 2 CH 3
2
2
2
(1.3 eq.) 1.0 eq NaI TBDMSO
88% > 99:1 Z:E
An alternative procedure for effecting the condensation of phosphonoacetates is
to carry out the reaction in the presence of lithium chloride and an amine such as diiso-
propylethylamine. The lithium chelate of the substituted phosphonate is sufficiently
acidic to be deprotonated by the amine. 262
Li + +
O O Li –
R N O O R′′CH O
P C 3
(R′O) 2 R′′CH CHCO R
OR (R′O) P C 2
2
CH 2 OR
C
H
256
A. Krotz and G. Helmchen, Liebigs Ann. Chem., 601 (1994).
257 K. Ando, Tetrahedron Lett., 36, 4105 (1995); K. Ando, J. Org. Chem., 63, 8411 (1998).
258
K. Ando, J. Org. Chem., 62, 1934 (1997); K. Ando, T. Oishi, M. Hirama, H. Ohno, and T. Ibuka, J.
Org. Chem., 65, 4745 (2000).
259
K. Kokin, J. Motoyoshiya, S. Hayashi, and H. Aoyama, Synth. Commun., 27, 2387 (1997).
260 S. Sano, K. Yokoyama, M. Shiro, and Y. Nagao, Chem. Pharm. Bull., 50, 706 (2002).
261 F. P. Touchard, Tetrahedron Lett., 45, 5519 (2004).
262
M. A. Blanchette, W. Choy, J. T. Davis, A. P. Essenfeld, S. Masamune, W. R. Roush, and T. Sakai,
Tetrahedron Lett., 25, 2183 (1984).