Page 196 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 196
168
Scheme 2.18. (Continued)
CHAPTER 2
O
Reactions of Carbon O
Nucleophiles with 10 k OH CSC H
2 5
Carbonyl Compounds CSC H OH
2 5
CH CHCH CH O
3
2
O CH
LiCl, (IPr) NEt 3
2
H )
P(OC 2 5 2 TBSO
TBSO
O O CH 3 O O 72%
CH 3
CH CH(OCH )
3 2
2
O CH
11 l CH CH(OCH ) O 3
3 2
2
N CH P(OCH ) CH LDA N
3 2
2
CH 3 O 3 CH 3 OPMB
O + O –78°C O CH
OPMB 3 CH 3
CH 3 CH 89% yield at
3
49% conversion
a. W. S. Wadsworth, Jr., and W. D. Emmons, Org. Synth., 45, 44 (1965).
b. R. J. Sundberg, P. A. Bukowick, and F. O. Holcombe, J. Org. Chem., 32, 2938 (1967).
c. W. S. Wadsworth, Jr., and W. D. Emmons, J. Am. Chem. Soc., 83, 1733 (1961).
d. J. A. Marshall, C. P. Hagan, and G. A. Flynn, J. Org. Chem., 40, 1162 (1975).
e. N. Finch, J. J. Fitt, and I. H. S. Hsu, J. Org. Chem., 40, 206 (1975).
f. M Mikolajczyk and R. Zurawski, J. Org. Chem., 63, 8894 (1998).
g. G. M. Stork and E. Nakamura, J. Org. Chem., 44, 4010 (1979).
h. K. C. Nicolaou, S. P. Seitz, M. R. Pavia, and N. A. Petasis, J. Org. Chem., 44, 4010 (1979).
i. M. A. Blanchette, W. Choy, J. T. Davis, A. P. Essenfeld, S. Masamune, W. R. Roush, and T. Sakai, Tetrahedron
Lett., 25, 2183 (1984).
j. G. E. Keck and J. A. Murry, J. Org. Chem., 56, 6606 (1991).
k. G. Pattenden, M. A. Gonzalez, P. B. Little, D. S. Millan, A. T. Plowright, J. A. Tornos, and T. Ye, Org. Biomolec.
Chem., 1, 4173 (2003).
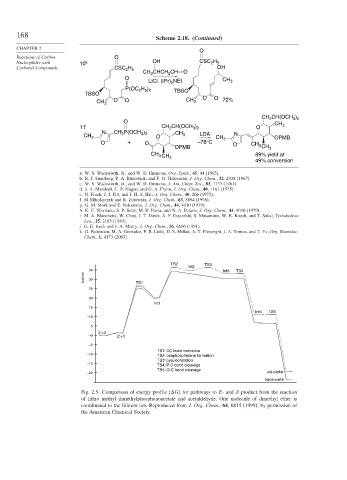
TS2 TS3
Int2
35 Int3 TS4
kcal/mol 30 TS1
25
20
Int1
15
Int4 TS5
–10
5
2′+3
–0 2′+3
–5
TS1: CC bond formation
–10 TS2: oxaphosphetane formation
TS3: pseudorotation
–15
TS4: P-C bond cleavage
TS5: O-C bond cleavage
–20 cis-olefin
trans-olefin
Fig. 2.5. Comparison of energy profile (
G) for pathways to E- and Z-product from the reaction
of lithio methyl dimethylphosphonoacetate and acetaldehyde. One molecule of dimethyl ether is
coordinated to the lithium ion. Reproduced from J. Org. Chem., 64, 6815 (1999), by permission of
the American Chemical Society.