Page 201 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 201
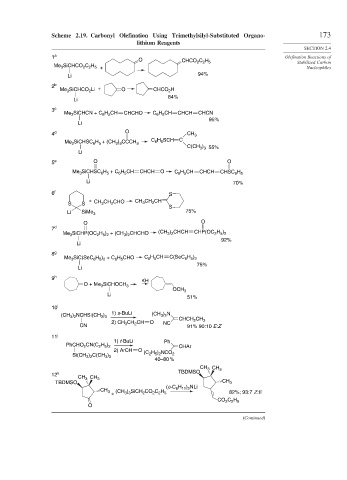
Scheme 2.19. Carbonyl Olefination Using Trimethylsilyl-Substituted Organo- 173
lithium Reagents
SECTION 2.4
1 a Olefination Reactions of
O
Stabilized Carbon
CHCO 2 C 2 H 5
+ Nucleophiles
Me 3 SiCHCO 2 C 2 H 5
Li 94%
2 b
Me 3 SiCHCO 2 Li + O CHCO 2 H
84%
Li
3 c
Me 3 SiCHCN + C 6 H 5 CH CHCHO C 6 H 5 CH CHCH CHCN
95%
Li
4 d O CH 3
C 6 H 5 SCH C
Me 3 SiCHSC 6 H 5 + (CH 3 ) 3 CCCH 3
C(CH 3 ) 3 55%
Li
5 e O O
Me 3 SiCHSC 6 H 5 + C 6 H 5 CH CHCH O C 6 H 5 CH CHCH CHSC 6 H 5
Li 70%
6 f S
+ CH 3 CH 2 CHO CH 3 CH 2 CH
S S
S
Li SiMe 3 75%
O O
7 d
Me 3 SiCHP(OC 2 H 5 ) 2 + (CH 3 ) 2 CHCHO (CH 3 ) 2 CHCH CHP(OC 2 H 5 ) 2
92%
Li
8 g
Me 3 SiC(SeC 6 H 5 ) 2 + C 6 H 5 CHO C 6 H 5 CH C(SeC 6 H 5 ) 2
75%
Li
9 h
KH
O + Me 3 SiCHOCH 3
OCH 3
Li
51%
10 i
1) s-BuLi (CH 3 ) 2 N
(CH 3 ) 2 NCHSi(CH 3 ) 3
2) CH 3 CH 2 CH O NC CHCH 2 CH 3
CN 91% 90:10 E:Z
11 j
1) t-BuLi Ph
PhCHO 2 CN(C 2 H 5 ) 2 CHAr
2) ArCH O
(C 2 H 5 ) 2 NCO 2
Si(CH 3 ) 2 C(CH 3 ) 3
40–80 %
CH 3 CH 3
12 k TBDMSO
CH 3 CH 3
TBDMSO CH 3
(c-C 6 H 11 ) 2 NLi
CH 3 (CH 3 ) 3 SiCH 2 CO 2 C 2 H 5 82%; 93:7 Z:E
+
CO 2 C 2 H 5
O
(Continued)